Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 n.6 Johannesburg Jun. 2018
http://dx.doi.org/10.17159/2411-9717/2018/v118n6a7
INFACON XV: INTERNATIONAL FERRO-ALLOYS CONGRESS 2018
Thermodynamic evaluation of Sr-containing Si metals and silicate melts for Si-Sr alloy production
K. TangI; L.K. JakobssonI; K. HildalII
ISINTEF Industry, Norway
IIElkem Technology, Norway
SYNOPSIS
Reliable thermodynamic descriptions of the Si-based Sr-Al-Ca-Fe-Mg metal system and SiO2-SrO-CaO-MgO-Al2O3 oxide system are essential for the understanding and optimizing the production of Si-Sr alloys. Phase relations of all SrO containing binary and ternary subsystems were evaluated and thermodynamically modelled based on the existing literature data. Phase equilibria in the SiO2-SrO-CaO-MgO-Al2O3 higher-order oxide system were thus possible to calculate by the CALPHAD method. A thermodynamic description of the Si-Sr-Al-Ca-Fe-Mg metal system was developed based on assessment of all binary systems as well as all Si containing ternary subsystems. The newly developed thermochemical databases can be used to calculate the equilibrium distribution of Sr between metal and slag. The theoretical Sr distribution maps for both pure Si and 75FeSi alloys in different SrO-containing slags are reported.
Keywords: thermodynamic evaluation, Si-Sr-Al-Ca-Fe-Mg metal, SiO2-SrO-CaO-MgO-Al2O3 oxide, Sr equilibrium distribution.
Introduction
Strontium distribution equilibria between the Si-based metals and SrO-containing silicate melts are of great importance for the development of new ferrosilicon products. To describe the complex phase equilibria between the Sr-containing Si metals and silicate slags, thermodynamic descriptions of the Sr-containing metal and oxide phases are essential. This paper summarizes our recent work on thermodynamic modelling of the Si-rich Si-Sr-Al-Ca-Fe-Mg metal and SiO2-SrO-CaO-MgO-Al2O3 oxide systems.
Thermochemical descriptions of the above metal and oxide phases were input into databases for the FactSage™ software package. The model calculations for the phase equilibrium relations in the SiO2-SrO-Al2O3 ternary oxide system were verified by laboratory experiments. The metal/slag strontium distribution equilibria at elevated temperatures were then evaluated thermodynamically. The present equilibrium simulations can serve as a 'map' for the metallurgist to design and optimize Si-Sr ferroalloy production.
Thermodynamic assessments
The metal system
Thermodynamic descriptions of the liquid and solid Si-based Si-Al-Ca-Sr-Fe-Mg alloy phases have been set up based mainly on the experimental data available in the literature. The compound energy formalism is applied to liquid and all the mixture phases as well as stoichiometric compounds.
The six-element metal system contains 15 binary, 20 ternary, 15 quaternary, and 6 quinary subsystems. Since the metal system is in the Si-rich domain, the Si-free ternary and higher-order subsystems are not considered in the present study. To ensure the reliability of the model calculation, all 15 binary subsystems were modelled to cover the whole composition range, and temperatures from the liquid phase to the sub-liquidus solid phases.
The calculated Sr-Si binary phase diagram, based on the assessment of Li et al. (2011), is shown in Figure 1. Experimental phase equilibrium data reported by Palenzona and Pani (2004) and Rygalin et al. (2010) is also shown in the diagram. The assessment emphasized the eutectic and peritectic reaction temperatures and compositions.

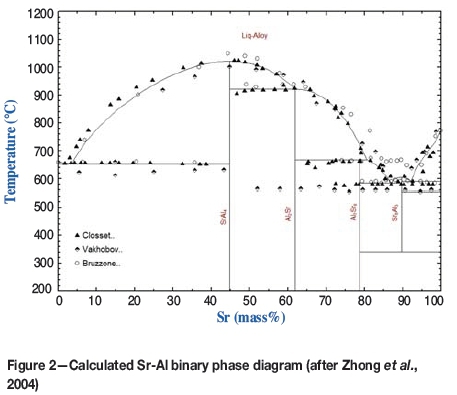
The experimental Sr-Al phase equilibria reported by Closset and Gruzleski (1982), Vakhobov, Eshonov, and Dzhurayev (1979), and Bruzzone and Merlo (1975) can be reproduced using the model parameters assessed by Zhong et al. (2004). The calculated phase diagram is shown in Figure 2.
The Ca-Sr phase diagram, assessed by the present authors based on the experimental liquidus and solidus data by Schottmiller, King, and Kanda (1958) is shown in Figure 3. These three binary subsystems, together with the Sr-Fe binary, are of great importance for the phase equilibria and elemental distributions of Sr-containing alloys.

There exist 10 Si-containing ternary subsystems which are also very important for the present thermodynamic descriptions, as shown in Table I. Only a few ternary systems have been thermodynamically assessed in the literature. Since the Sr concentrations in pure Si and FeSi alloys are in the range of dilute solution, i.e. < 5 wt%, ternary and higherorder contributions to the melts will not be significant. This means that the present database can represent thermochemical properties of the Si-based Sr-Al-Ca-Fe-Mg alloys at elevated temperatures.
The calculated Al-Al2Si2Sr pseudo-binary phase diagram is shown in Figure 4. In this ternary alloy system, the model calculation can reproduce the experimental phase boundaries (Sato et al., 1985) quite well. The agreement between the calculated and measured phase equilibria by Vakhobov, Eshonov, and Dzhurayev (1979) is also satisfactory in the low-Sr composition domain.

Thermodynamic model calculations for the phase equilibria in the Si-Mg-Sr ternary as well as in the Si-Al-Ca-Fe quaternary system were also examined with the experimental data in the literature. For the sake of simplicity, they are not presented here.
Thermodynamic properties of the Si-Sr-Al-Ca-Fe-Mg system are all extrapolated from the above assessed binaries and some ternaries, as well as Si-Al-Ca-Fe quaternary subsystems.
Oxide systems
The SiO2-CaO-Al2O3-SrO-MgO oxide system consists of 10 binaries and 10 ternaries. Unlike the Si-rich metals, the slags vary over a large composition range. It is thus necessary to evaluate whole binary and ternary oxide subsystems in their entirety. To define the oxide equilibrium contents, both the liquid slag and solid oxide phases need to be correctly modelled. In the present work, the molten slag phase was modelled using the modified quasichemical solution model (Pelton and Blander, 1986). Solid non-stochiometric phases, for instance the monoxide phases, were modelled using the compound energy formalism.
Thermodynamic models of the SrO-free SiO2-CaO-Al2O3-MgO oxide system can be found in the literature. Four SrO-containing binary and three SrO-containing ternary subsystems were modelled in the present work. Table II lists the binary oxide subsystems used.

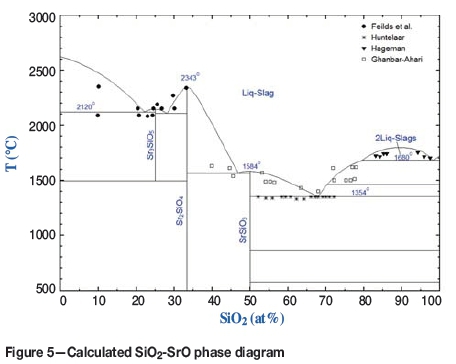
The liquid SiO2-SrO phase was assessed based on the phase equilibrium data available in the literature (Fields, Dear, and Brown, 1972; Hageman and Oonk, 1986; Huntelaar, Cordfunke, and Ouweltjes, 1992; Ghanbari-Ahari and Brett, 1988). All solid mixture phases in the present work are treated as stoichiometric compounds.
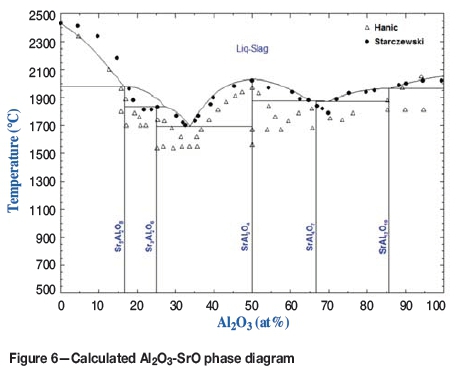
The experimental phase equilibria data related to liquid SrO-Al2O3 slag were mainly based on the experimental results of Hanic, Chemekova, and Udalov (1979) and Starczewski (1964). The present assessment was based on the experimental data of Starczewski (1964). The calculated SrO-Al2O3 phase diagram is shown in Figure 6. The model calculations fit the experimental equilibrium data by Starczewski (1964), since his data fits well to the SrO-SiO2-Al2O3 ternary phase equilibria.
Phase equilibria in the SrO-SiO2-Al2O3 system are of crucial importance for the determination of the Sr distribution between slag and metal. There are three ternary compounds reported by Dear (1957), namely Si2SrAl2O8, Si2Sr6Al18O37, and SiSr2Al2O7. Thermodynamic properties of these compounds were also reported in the literature (Lapina, Semenov, and Khodakovskii, 1989; Massazza and Sirchia, 1959). Based on the literature data, properties of these ternary compounds were estimated.
The calculated liquidus projection of the SiO2-SrO-Al2O3 ternary oxide system is shown in Figure 7. It is seen that this ternary system is similar to the well-known SiO2-CaO-Al2O3 ternary system. Two SrO-containing compounds precipitate in the middle of the composition range, where the slags exhibit low smelting temperatures.
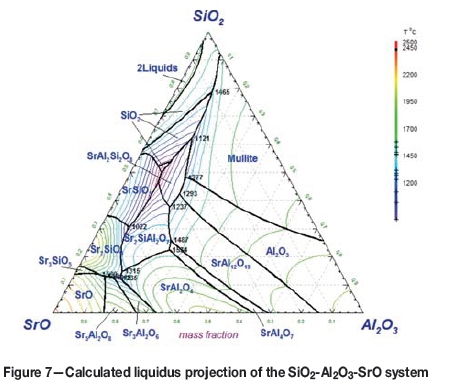
For the sake of simplicity, detailed descriptions of the experimental data as well as model assessment will not be presented here. The information related to other ternary oxide subsystems is summarized in Table III.

Slag/metal equilibria
The slag/metal Sr partition equilibria are the most interesting aspect of the present study. The partition of Sr between Si-Sr metals and SrO-SiO2 binary slags at different temperatures is shown in Figure 8. The Sr distribution equilibria between 75FeSi alloys and SrO-SiO2 binary slags at 1600°C are also shown in the same diagram.
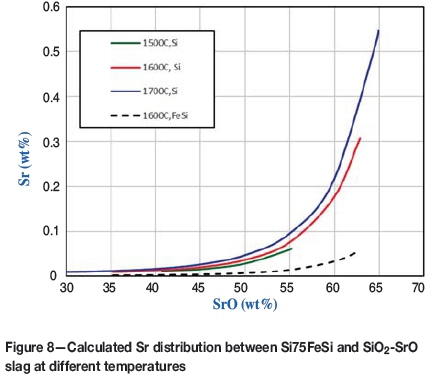
It is notable that the Sr contents in 75FeSi are about 10 times lower than those in Si metals. This is due to the strong positive interaction between Fe and Sr, which can also be observed by the immiscibility of Fe and Sr.
Figure 9 shows the iso-Sr composition contours for pure Si in equilibrium with the ternary SiO2-SrO-Al2O3 slags at 1600°C. The amphoteric property of Al2O3 is rather obvious, i.e. Al2O3 acts as a basic oxide in the SiO2-rich area and an acid oxide in the SrO-rich domain. Figure 10 shows the similar equilibrium iso-Sr contours for the same system at 1700°C.


By comparing Figure 10 to Figure 9, we see that temperature does not play a key role in the Sr partition equilibria in this ternary slag system. This means that the silicothermic reduction of SrO is relatively independent of temperature. However, slag composition plays a vital role in the Sr contents in Si metals, in particular for the higher SrO slags. By increasing the SrO content in the slag from 55 wt% to 60 wt%, the final equilibrium Sr content in Si will increase from around 6-7 wt% to 18-31 wt% at 1600-1700°C.
The present thermodynamic models can also be used to evaluate the solidification of Si/75FeSi alloys, smelting behaviour of SrO-containing slags, as well as materials and energy balances during metallurgical processes. This will be discussed in our next publication.
Conclusions
Thermodynamic descriptions of the Si-based Sr-Al-Ca-Fe-Mg metal and SiO2-SrO-CaO-MgO-Al2O3 oxide systems have been established and implemented in the commercial software package FactSage. Thermodynamic modelling of the Si-based Si-Sr-Al-Ca-Fe-Mg metal system was developed based on assessments of all binary systems as well as the Si-Sr-Al, Si-Sr-Mg, Si-Al-Mg ternary, and Si-Al-Ca-Fe quaternary subsystems.
Phase relations in the SiO2-SrO, SrO-Al2O3 binary as well as the SiO2-SrO-Al2O3, SiO2-SrO-CaO, and SiO2-SrO-MgO ternary system were evaluated and thermodynamically modelled based on existing literature data. Phase equilibria in the SiO2-SrO-CaO-MgO-Al2O3 higher-order oxide system were thus possible to calculate by the CALPHAD method.
The thermochemical databases have been applied to evaluate the Sr distribution equilibria between pure Si and SiO2-SrO slags as well as FeSi and SiO2-SrO slags. In addition, the equilibrium distribution of Sr between pure silicon and SiO2-SrO-CaO-Al2O3 slags was calculated. It is found that the Sr contents in Si metals are much higher than those in 75FeSi alloys. The Sr contents in Si metals will depend strongly upon the slag composition, and weakly upon the temperature.
Acknowledgements
This work was supported by the Research Council of Norway through the project CoRReSi (project number: 235159/O30).
References
Anglezio, J.C., Servant, C., and Ansara, I.1994a. Contribution to the experimental and thermodynamic assessment of the Al-Ca-Fe-Si system. I.Al-Ca-Fe, Al-Ca-Si, Al-Fe-Si and Ca-Fe-Si systems. Calphad - Computer Coupling of Phase Diagrams and Thermochemistry, vol. 18. pp. 273-309. [ Links ]
Anglezio, J.C., Servant, C., and Ansara, I. 1994b. A study of the Si-rich domain of the Al-Ca-Fe-Si quaternary system. Calphad - Computer Coupling of Phase Diagrams and Thermochemistry, vol. 18. pp. 311-318. [ Links ]
Bruzzone, G. and Merlo, F. 1975. The strontium-aluminium and barium-aluminium systems. Journal of the Less Common Metals, vol. 39. pp. 1-6. [ Links ]
Closset, B. and Gruzleski, J.E. 1982. Structure and properties of hypoeutectic Al-Si-Mg alloys modified with pure strontium. Metallurgical Transactions A, vol. 13. pp. 945-951. [ Links ]
Dear, P.S. 1957. Sub-liquidus equilibria for the ternary system SrO-Al2O3-SiO2. Virginia Polytechnic Institute, Blacksburg, VA. [ Links ]
Decterov, S., Jung, I-H., and Pelton, A.D. 2002. Thermodynamic modeling of the FeO-Fe2O3-MgO-SiO2 system. Journal of the American Ceramic Society, vol. 85. pp. 2903-3910. [ Links ]
Duo, Y., Zhao, J.R., Zhang, C., Chen, H.L., and Zhang, L.J. 2007. Thermodynamic modeling of the Fe-Mg-Si system. Journal of Mining and Metallurgy Section B - Metallurgy, vol. 43. pp. 39-56. [ Links ]
Effenberg, G. and Ilyenko, S. 2004. Al-Ca-Si (aluminium - calcium - silicon). Light Metal Systems. Part 1: Selected Systemsfrom Ag-Al-Cu to Al-Cu-Er. Springer, Berlin/Heidelberg. [ Links ]
Eriksson, G. and Pelton, A.D. 1993. Critical evaluation and optimization of the thermodynamic properties and phase-diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 systems. Metallurgical Transactions B - Process Metallurgy, vol. 24. pp. 807-816. [ Links ]
Feufel, H., Gödecke, T., Lukas, H.L., and Sommer, F. 1997. Investigation of the Al-Mg-Si system by experiments and thermodynamic calculations. Journal of Alloys and Compounds, vol. 247. pp. 31-42. [ Links ]
Fields, J., Paul, M., Dear, S., and Brown, J.J. 1972. Phase equilibria in the system BaO-SrO-SiO2. Journal of the American Ceramic Society, vol. 55. pp. 585-588. [ Links ]
Ghanbari-Ahari, K. and Brett, N.H. 1988. Phase equilibria and microstructure in the system zirconia-magnesia-silica-strontia. Part 2: The ternary system magnesia-silica-strontia. British Ceramic Transactions, vol. 87. pp. 103-106. [ Links ]
Gil-Santos, A., Moelans, N., Hort, N., and van der Biest, O. 2016. Identification and description of intermetallic compounds in Mg-Si-Sr cast and heat-treated alloys. Journal of Alloys and Compounds, vol. 669. pp. 123-133. [ Links ]
Gröbner, J., Chumak, I., and Schmid-Fetzer, R. 2003. Experimental study of ternary Ca-Mg-Si phase equilibria and thermodynamic assessment of Ca-Si and Ca-Mg-Si systems, Intermetallics, vol. 11. pp. 1065-74. [ Links ]
Hageman, V.B.M. and Oonk, H.A.J. 1986. liquid immiscibility in the SiO2 + MgO, SiO2 + SrO, SiO2 + La2O3, and SiO2 + Y2O3 systems. Physics and Chemistry of Glasses, vol. 27. pp. 194-98. [ Links ]
Hanic, F., Chemekova, T.Y., and Udalov, Y.P. 1979. Strontium oxide-alumina system. Russian Journal of Inorganic Chemistry (Zhurnal Neorganicheskoi Khimii), vol. 24. pp. 471-475. [ Links ]
Huntelaar, M.E., Cordfunke, E.H.P., and Ouweltjes, W. 1992. The standard molar enthalpies of formation of SrSiO3(s) and Sr2SiO4(s). Journal of Chemical Thermodynamics, vol. 24. pp. 139-143. [ Links ]
Janz, A. and Schmid-Fetzer, R. 2009. Thermodynamics and constitution of Mg-Al-Ca-Sr-Mn Alloys: Part I. Experimental investigation and thermodynamic modeling of subsystems Mg-Ca-Sr and Al-Ca-Sr. Journal of Phase Equilibria and Diffusion, vol. 30. pp. 146-156. [ Links ]
Jung, I.H., Decterov, S.A., and Pelton, A.D. 2004a. Critical thermodynamic evaluation and optimization of the MgO-Al2O3, CaO-MgO-Al2O3, and MgO-Al2O3-SiO2 systems. Journal of Phase Equilibria and Diffusion, vol. 25. pp. 329-345. [ Links ]
Jung, I-H., Decterov, S.A., and Pelton, A.D. 2004b. Critical thermodynamic evaluation and optimization of the MgO-Al2O3, CaO-MgO-Al2O3, and MgO-Al2O3-SiO2 systems. Journal of Phase Equilibria and Diffusion, vol. 25. pp. 329-345. [ Links ]
Lapina, I.V., Semenov, Y.V., and Khodakovskii, I.L. 1989. Thermodynamic properties of calcium-, strontium-, and barium feldspars based on calorimetric data. Geokhimiya, vol. 7. pp. 1033-1032. [ Links ]
Li, K., Liu, S., Sha, C., and Du, Y. 2011. A thermodynamic reassessment of the Si-Sr system. Calphad, vol. 35. pp. 594-600. [ Links ]
Massazza, F. and Sirchia, E. 1959. Equilibriums at the temperature of fusion in the ternary system SrO-Al2O3-CaO. Annali di Chimica, vol. 49. pp. 1352-1370. [ Links ]
Palenzona, A. and Pani, M. 2004. The phase diagram of the Sr-Si system. Journal of Alloys and Compounds, vol. 373. pp. 214-219. [ Links ]
Pelton, A.D. and Blander, M. 1986. Thermodynamic analysis of ordered liquid solutions by a modified quasichemical approach-Application to silicate slags. Metallurgical TransactionsB, vol. 17. pp. 805-815. [ Links ]
Rokhlin, L.E.L. and MSIT® Materials Science International Team. 2000. Ca-Mg-Si ternary phase diagram evaluation · Phase diagrams, crystallographic and thermodynamic data: MSI Eureka report 10.16786.1.7. Springer Materials. http://materials.springer.com/msi/docs/sm_msi_r_10_016786_01 [ Links ]
Rokhlin, L. and Dobatkina, T. 2001. Mg-Si-Sr ternary phase diagram evaluation. Ternary Evaluations. Effenberg, G. (ed.). MSI, Materials Science international Services, Stuttgart. [ Links ]
Rygalin, B., Prokofieva, V., Pavlova, L., and okolov, Y.E.S. 2010. The Si-Sr and Si-Ba phase diagrams over the Si-rich composition range. Calphad, vol. 34. pp. 196-199. [ Links ]
Sato, E., Kono, M., Sato, I., and Watanabe, H. 1985. Study on the phase diagram of Al-Si-Sr ternary alloy system. Journal of Japan Institute of Light Metals, vol. 35. pp. 71-78. [ Links ]
Schottmiller, J.C., King, A.J., and Kanda, F.A. 1958. The calcium-strontium metal phase system. Journal of Physical Chemistry, vol. 62. pp. 1446-1449. [ Links ]
Starczewski, M. 1964. Treatise on solid state reactions in the ternary system SrO-Al2O3-SiO2. Zeszyty Naukowe PolitechnikiSlaskiej, Chemia, vol. 22. pp. 5-75. [ Links ]
Tang, Y., Du, Y., Zhang, L.J., Yuan, X.M., and Kaptay, G. 2012. Thermodynamic description of the Al-Mg-Si system using a new formulation for the temperature dependence of the excess Gibbs energy. Thermochimica Acta, vol. 527. pp. 131-142. [ Links ]
Vakhobov, A.V., Eshonov, K.K, and Dzhurayev, T.D. 1979. The Al-Sr-Nd phase diagram. Russian Metallurgy. pp. 167-172. [ Links ]
Vakhobov, A.V., Ganiev, I.N., and Djuraev, T.D. 1975. IZV. Akad. Nauk. USSR Met., 15. [ Links ]
Wu, P., Eriksson, G., and Pelton, A.D. 1993. Critical evaluation and optimization of the thermodynamic properties and phase-diagrams of the CaO-FeO, CaO-MgO, CaO-MnO, FeO-MgO, FeO-MnO, and MgO-MnO sytems. Journal of the American Ceramic Society, vol. 76. pp. 2065-2075. [ Links ]
Zhong, Yu., Wolverton, C,. Austin Chang, Y., and Liu, Z-K. 2004. A combined CALPHAD/first-principles remodeling of the thermodynamics of Al-Sr: unsuspected ground state energies by 'rounding up the (un)usual suspects'. Acta Materialia, vol. 52. pp. 2739-2754. [ Links ]














