Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 n.5 Johannesburg May. 2018
http://dx.doi.org/10.17159/2411-9717/2018/v118n5a10
PAPERS OF GENERAL INTEREST
Effect of cooling conditions on the leachability of chromium in Cr2O3-containing steelmaking slag
Y. YuI; D. WangI; J. LiI, II; M. LiIII; Z. XueI
IState Key Laboratory of Refractories and Metallurgy, Wuhan University of Science and Technology, China
IIKey Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education, Wuhan University of Science and Technology, China
IIIYonggang Group Co.,LTD, China
SYNOPSIS
The effects of temperature and atmosphere during cooling on the leachability of chromium from stainless steel slag were investigated. Experiments were performed under an oxidizing atmosphere in a muffle furnace and in a reducing atmosphere in an induction furnace. The slags were subjected to leaching tests using the standard procedure prEN12457-2. Results indicated that an oxidizing atmosphere enhances the leachability of chromium and that the extent of leaching increases with temperature. A reducing atmosphere tends to suppress the leaching of chromium. Differences between the chromium leached from the samples cooled under different atmospheres revealed that it is difficult for CO to completely reduce chromium Cr6+ to Cr3+ once hexavalent chromium has been generated. Therefore, it is necessary to utilize a neutral or reducing atmosphere for cooling the slag so as to prevent the formation of hexavalent chromium.
Keywords: atmosphere, oxidation, hexavalent chromium, stainless steel slag, chromium leaching.
Introduction
Stainless steel slags are generated in electric arc furnace (EAF), argon oxygen decarburization (AOD) furnace, and vacuum oxygen decarburization furnace operations. The transition metal chromium (Cr), which is an essential alloying element in stainless steel, is prone to oxidation because of its high reactivity during melting (Li et al., 2013), and chromium oxides float in the molten slag phase. Trivalent and hexavalent chromium are the most common oxidation states in chromium compounds. Trivalent chromium is the most stable state under normal atmospheric conditions, whereas hexavalent chromium is toxic because of its strong oxidative property (Zhong et al., 2015). The leaching of chromium from stainless steel slags can lead to economic and ecological issues (Loock-Hattingh et al., 2015). Therefore, it is imperative to examine the mechanism involved in the leaching of chromium and design some effective treatments to stabilize chromium in slags.
Several studies have reported the leaching and stabilization of chromium (Engstrom, 2010; Durinck et al., 2008; Albertsson, 2011; Tae and Morita, 2017; Gelfi, Cornacchia, and Roberti, 2010; Song and Garbers-Craig, 2016; Du Preez et al, 2017). Engstrom (2010) and Albertsson (2011) reported that the leachability of chromium depends mainly on the distribution of chromium in the slag. Stainless steel slag comprises various minerals. Some of these minerals, such as merwinite (Ca3MgSi2O8), larnite (Ca2SiO4), akermanite (Ca2MgSi2O7), and gehlenite (Ca2Al2SiO7), are slightly soluble in water. In contrast, other minerals, such as wüstite, and spinel, are resistant to dissolution and oxidation. The MgCr2O4 spinel phase, i.e., magnesiochromite, in the slag is known to be important for controlling the leaching properties of chromium from the slag (Pillay, Von, and Petersen, 2003). Garcia-Ramos et al., (2008) investigated the immobilization of chromium in CaO-SiO2-Cr2O3-Al2O3-MgO synthetic slags by MgO. Their results indicated that MgO-based slag exhibits the lowest chromium leachability, owing to the immobilization of chromium in MgO-spinel. Engström et al., (2010) and Peter et al., (2010) developed a new method for treating EAF slag obtained from stainless steelmaking. The agents that enhance the formation of the spinel solid solution are added into a transfer ladle during tapping of the steel and slag. The effect of the additions on spinel formation can be described as follows:
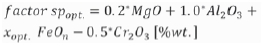
where xopt. depends on the oxidation state of the EAF slag.
For EAF slags with high values of spopt., the leachable chromium content is almost negligible. These studies focused on the suppression of chromium leaching by adjusting the composition. Few studies have been reported regarding the effect of atmosphere on the leaching of chromium during cooling of stainless steelmaking slag. Considering the production of ferrochrome alloys from chromite ores, Beukes, Dawson, and Zyl (2010) reported that the generation of Cr(VI) is related to the presence of oxygen at a high temperature.
In this study, to increase understanding of the mechanism involved in the leaching of chromium from stainless steel slags, the effects of atmosphere and temperature on the leachability of chromium during the cooling of liquid slag were investigated by experiments performed in muffle and induction furnaces.
Experimental
Raw material
Table I summarizes the chemical composition of the slags obtained from the stainless-steelmaking process. Samples S1 and S2, which were generated in an AOD furnace, had an extremely high calcium oxide content, 61.54 wt% and 54.46 wt%, respectively, whereas the chromium oxide content in both slags was quite low, only 0.19 wt% and 0.51 wt%, respectively. Sample S3, the EAF slag, had the lowest basicity (CaO/SiO2), albeit with a fairly high Cr2O3 content (5.78 wt%).
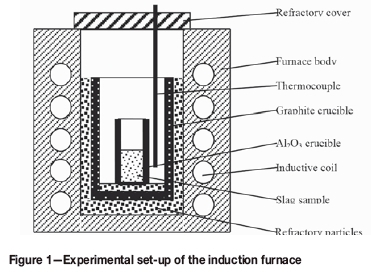
Experimental set-up and procedure
The experimental set-up included the muffle and induction furnaces (Figure 1). The maximum temperature of the muffle furnace was 1200°C. A graphite crucible was used as the heating element for the induction furnace, with a maximum power of 60 kW and frequency of 3 kHz. Figure 2 shows the experimental procedure. First, 270 g of each of the slag samples was crushed to less than 4 mm. Then, 90 g of particles was used for the leaching test, which was conducted according to the European standard leaching procedure prEN12457-2. The remaining 180 g was charged in an Al2O3 crucible and heated in the muffle furnace at 600°C and 1000°C for 180 minutes under air. Then, 90 g of the oxidized slag obtained from the muffle furnace in the previous step was used in the leaching test, and the remaining 90 g was heated in the induction furnace at the same temperature as in the muffle furnace for 180 minutes. Finally, the reduced slags were subjected to the leaching test. The leachates obtained from the tests were analysed using inductively coupled plasma emission spectrometry (ICP-OES, IRIS Advantage ER/S), and HSC Chemistry 5.1 was employed to perform thermodynamic calculations for some reactions.
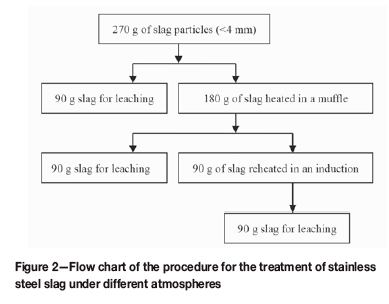
The standard procedure is in compliance with the leaching of granular waste materials and sludge, and a one-stage batch test is conducted at a liquid-to-solid ratio of 10 L/kg for materials with a particle size of less than 4 mm. The complete leaching procedure included the following steps:
(i) Add 90 g of slag into a 1 L polypropylene bottle
(ii) Add 900 mL of deionized water to the bottle
(iii) Place the capped bottle in a rotary agitator (GGC-D, Guohuan Institute of High-tech Automation)
(iv) Agitate for 24 ± 0.5 hours at a rate of 10 r/min
(v) Filter the mixture using a vacuum filtration device (TTGM, 2000 mL, Lianlink).
Atmosphere in an induction furnace
Notably, a graphite crucible is used as the heating element in the induction furnace; thus, the interactions between O2, CO2, and CO occur at a high temperature (Huang, 2006).
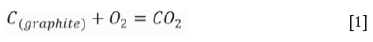

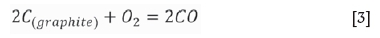
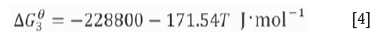
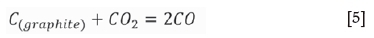
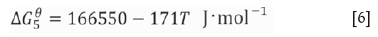


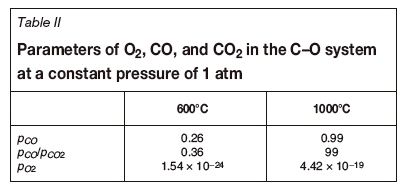
The partial pressures of O2, CO, and C2 are calculated at 600°C and 1000°C at the equilibrium state of the C-O system. A non-oxidizing atmosphere is present at high temperatures (Table II). CO was used for the reduction of slag. Niemelä , Krogerus, Oikarinen, (2004) and Kleynhans et al. (2016) have revealed that CO does not reduce Cr2O3. The possibility of the reduction of chromium from the hexavalent to the trivalent state by CO is discussed in the following sections.
Experimental results
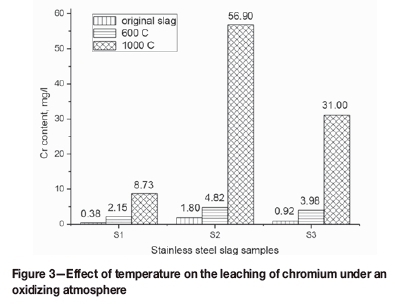
The slag samples were heated at 600°C and 1000°C in the muffle furnace for 180 minutes and then leached according to the standard leaching procedure prEN12457-2. Higher chromium concentrations were observed in the leachates of the oxidized slags compared with those in the original slag samples. The chromium concentration increases with temperature (Figure 3). After sample S1 had been subjected to oxidation at 600°C and 1000°C, the concentrations of chromium in the leachates were 466% and 2197% greater than those of the original slag, respectively. The corresponding values for sample S2 increased to 4.82 mg/L and 56.90 mg/L. These values are considerably greater than that of the original slag (1.80 mg/L). Moreover, the concentrations of chromium in the leachates of sample S3 increased by 333% and 3270% compared with the original slag.
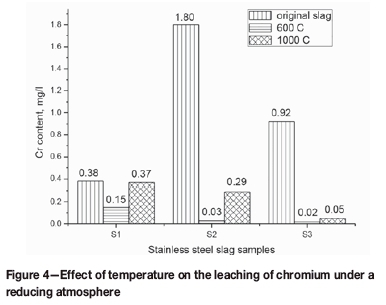
After reduction of the slag in the induction furnace, the content of chromium in the leachate decreases in comparison with that in the original slag (Figure 4). The three slag samples exhibited the same tendency: the highest chromium content in the leachate is observed for the original slag, whereas the lowest concentration chromium is observed for the slag reduced at 600°C. When S1 was oxidized at 600°C in the muffle furnace and then reduced at 600°C in the induction furnace, the leachate chromium content decreased from 2.15 to 0.15 mg/L. When the sample was processed at 1000°C following the same procedure, the concentration of chromium in the leachate fell from 8.73 to 0.37 mg/L. The concentrations of leached chromium for the reduced samples of S2 and S3 were dramatically less than those observed for the corresponding oxidized samples. The decrease is greater for reduction at 1000°C than at 600°C. For example, the concentrations of chromium in the leachate from the reduced samples of slag S3 were 0.61% and 0.15% of those of the samples oxidized at 600°C and 1000°C, respectively. Furthermore, the concentrations of chromium in the leachates collected from the samples reduced at 1000°C were always greater than those at 600°C.
Discussion
Effect ofatmosphere on chromium leaching
Thermodynamically, the amount of chromium dissolving into water from the stainless steel slag depends on the CaCrO4 content (Lee and Nassaralla, 1997), which is expressed by the following reactions:
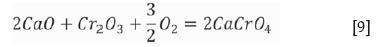
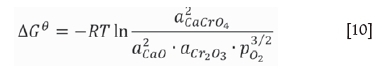

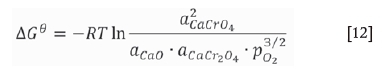
where a is the activity and p is the partial pressure. When pure solid CaO, Cr2O3, CaCr2O4, and CaCrO4are chosen as the standard states of activities, the standard Gibbs energy of formation for solid CaCrO4 is expressed as follows:

The slags were heated in the muffle furnace under air; thus, the partial pressure of oxygen was constant at 0.21. According to Equation [13], the standard Gibbs energy changes of reactions [9] and [11] can be expressed as a function of temperature, namely:

Hence, the treatment of slag in the muffle furnace enhances the formation of CaCrO4, and a high temperature may also favour the reactions. The concentration of chromium in the leachate therefore increases as a result of heating in the muffle furnace, and it increases with temperature.
As stated in the section 'Atmosphere in an induction furnace', the atmosphere in the induction furnace maintains strong reducibility. The data shown in Table II revealed that the partial pressure of oxygen is less than 4.42 χ 10-19 at a temperature of less than 1000°C, and it increases with temperature in the induction furnace. The soluble component, CaCrO4, will decompose according to reactions [15] and [16]:
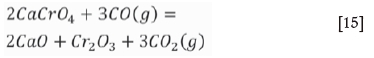
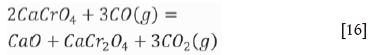
According to thermodynamic calculations performed using the FactSage 7.0 thermodynamic database, the standard Gibbs energy change of Equation [15] is expressed as follows:

Consequently, CaCrO4 formed in the muffle furnace can be reduced by CO produced by the interaction between graphite and air in the induction furnace, and the chromium contained in the slags transforms from the soluble state (Cr6+) into the stable states (Cr2O3 and CaCr2O4), leading to a significant decrease in the amount of chromium leached.
Moreover, the difference between the amounts of chromium dissolved from the three slags can be interpreted in terms of kinetics. The oxidation and reduction of chromium are gas-solid reactions, for which the interface is known to be a key factor. Pillay, Von, and Petersen, (2003) reported that the oxidation of a mixture of CaO and Cr2O3 supposedly occurs at the boundaries between CaO and Cr2O3 via the diffusion of oxygen along the particle boundaries, whereas that of Cr3+ occurs across the boundaries, leading to the formation of CaCrO4. In the furnace slags in which CaO and Cr2O3 would form a solid solution, oxidation possibly occurs at the exposed surface of particles containing this type of solid solution.
The chromium content dominates the distribution on the surface of the slag particles. Hence, S2 exhibits a higher amount of dissolved chromium compared with S1 because of the higher chromium content. However, S3 exhibits the highest chromium concentration, and the amount of dissolved chromium is less than that in S2 after treatment in a muffle furnace at 600°C and 1000°C (Figure 3). This discrepancy can be explained by the mode of occurrence of chromium in the furnace slags. Notably, chromium exists mainly as a solid solution, such as merwinite, akermanite, and matrix, which is related to the extremely low content in the AOD slag. However, for the EAF slag, the majority of the chromium would occur in spinel minerals, such as MgCr2O4 and FeCr2O4, which are thought to be resistant to oxidation and dissolution in water (Samada, Miki, and Hino, 2011).
Effect of temperature on chromium leaching
Notably, temperature has a considerable effect on the dissolution of chromium in the lixiviant (Figures 3 and 4). At a high temperature, a large amount of CaCrO4 is formed in the muffle furnace, which is reduced by CO in the induction furnace. These reactions can be explained by Arrhenius's Law, as shown in Equation [18] (Hua, 2004), indicating that either the increase in temperature or decrease in the activation energy can increase the reaction rate.

where k is the reaction rate, A is the pre-exponential factor, E is the activation energy, and R is the universal gas constant.
Nevertheless, the formation of CaCrO4 is restricted by temperature. Lee and Nassaralla (1998) reported that the formation of hexavalent chromium (Cr6+) in a magnesite-chrome refractory is affected by the reaction temperature and chemical composition. According to the CaO-Cr2O3 phase diagram shown in Figure 5 (Lee and Nassaralla, 1997), the concentration of Cr6+ increases at a temperature below the critical point of 1022°C. Hexavalent chromium is thought not to exist in the liquid slag, indicating that Cr6+ is formed during cooling. Hence, the neutral or reducing atmosphere during cooling possibly suppresses the formation of Cr6+ and the subsequent leaching of chromium.
In addition, the difference in the chromium concentration in the leachates shown in Figure 4 indicated that once hexavalent chromium is formed, it is difficult to completely reduce Cr6+ to Cr3+, suggesting that it is necessary to utilize a protective atmosphere for the cooling of slag to inhibit the formation of Cr6+.
Conclusions
To further understand the mechanism involved in the leaching of chromium from stainless steel slags, the effects of atmosphere and temperature on the leaching of chromium from slags were investigated at temperatures of 600°C and 1000°C in a muffle furnace under an oxidizing atmosphere and an induction furnace under a reducing atmosphere, and an efficient method for suppressing the leachability of chromium was identified.
Chromium can be oxidized to the hexavalent state by oxygen, and a high amount of chromium can leach into water at a high temperature under an oxidizing atmosphere. After the oxidation of sample S1 at 600°C and 1000°C, the concentrations of chromium were respectively 466% and 2197% these values are greater than that of the original slag.
Under a reducing atmosphere, CaCrO4 can be reduced to calcium oxide and chromium oxide by CO, leading to a decrease in the chromium concentration in the leachates. After sample S2 was first oxidized and then reduced at 1000°C, the concentration of leached chromium decreased from 56.90 to 0.29 mg/L. Once hexavalent chromium was formed, it was difficult to completely reduce to Cr3+. Thus, a neutral or reducing atmosphere can favour the suppression of chromium leaching.
Acknowledgement
The research was supported by National Natural Science Foundation of China (No.51404173), Hubei Provincial Natural Science Foundation (No. 2016CFB579), China Postdoctoral Science Foundation (No.2014M562073), and State Key Laboratory of Refractories and Metallurgy (No.2014QN21). We thank Dr Q. Yang and Professor A. Xu for assistance in preparing the samples.
References
Albertsson, G.J. 2011. Investigations of stabilization of Cr in spinel phase in chromium- containing slags. PhD thesis, Royal Institute of Technology, Sweden. [ Links ]
Beukes, J.P., Dawson, N.F., and Zyl, P.G.V. 2010. Theoretical and practical aspects of Cr(VI) in the South African ferrochrome industry. Journal of the Southern African Institute of Mining and Metallurgy, vol. 110, no. 12. pp. 743-750. [ Links ]
Du Preez, S.P., Beukes, J.P., Dalen, W.P.J.V., Zyl, P.G.V., Paktunc, D., and Loock-Hattingh, M.M. 2017. Aqueous solubility of Cr(VI) compounds in ferrochrome bag filter dust and the implications thereof. Water SA, vol. 43, no. 2. pp. 298-309. [ Links ]
Durinck, D., Engström, F., Arnout, S., Heulens, J., Jones, P.T., and Bo, B. 2008. Hot stage processing of metallurgical slags. Resources Conservation and Recycling, vol. 52, no. 10. pp. 1121-1131. [ Links ]
Engstrom, F. 2010. Mineralogical influence on leaching behaviour of steelmaking slags. PhD thesis, Luleá University of Technology, Sweden. [ Links ]
Engström, F., Pontikes, Y., Geysen, D., Jones, P.T., Bo, B., and Blanpain, B. 2011. Review: Hot stage engineering to improve slag valorisation options. Proceedings of the Second International Slag Valorisation Symposium: The Transition to Sustainable Materials Management, Leuven, Belgium, 18-20 April 2011. KU Leuven. pp. 231-251. [ Links ]
García-Ramos, Ε., Romero-Serrano, A., Zeifert, B., Flores-Sánchez, P., Hallen-López, M., and Palacios, E.G. 2008. Immobilization of chromium in slags using MgO and Al2O3. Steel Research International, vol. 79, no. 5. pp. 332-339. [ Links ]
Gelfi, M., Cornacchia, G., and Roberti, R. 2010. Investigations on leaching behavior of EAF steel slags. Proceedings of the 6th European Slag Conference, Madrid, 20-22 October 2010. Publication no. 5. EUROSLAG, Duisburg-Rheinhausen, Germany. pp. 1-13. [ Links ]
Hua, Y. 2004. Kinetics of Metallurgical Process. Metallurgical Industry Press, Beijing [in Chinese]. [ Links ]
Huang, X.H. 2006. Principle of Ferrous Metallurgy. Metallurgical Industry Press, Beijing. [ Links ]
Kleynhans, E.L.J., Neizel, B.W., Beukes, J.P., and Zyl, P.G.V. 2016. Utilization of pre-oxidized ore in the pelletized chromite pre-reduction process. Minerals Engineering, vol. 92. pp. 114-124. [ Links ]
Lee, Y. and Nassaralla, C.L. 1997. Minimization of hexavalent chromium in magnesite-chrome refractory. Metallurgical and Materials Transactions B, vol. 28, no. 5. pp.855-859. [ Links ]
Lee, Y. and Nassaralla, C.L. 1998. Formation of hexavalent chromium by reaction between slag and magnesite-chrome refractory. Metallurgical and Materials Transactions B, vol. 29, no. 2. pp. 405-410. [ Links ]
Li, J.L., Xu, A.J., He, D.F., Yang, Q.X., and Tian, N.Y. 2013. Effect of FeO on the formation of spinel phases and chromium distribution in the CaO-SiO2-MgO-Al2O3-Cr2O3 system. International Journal of Minerals, Metallurgy and Materials, vol.20, no.3. pp. 253-258. [ Links ]
Loock-Hattingh, M.M., Beukes, J.P., Zyl, P.G.V., and Tiedt, L.R. 2015. Cr(VI) and conductivity as indicators of surface water pollution from ferrochrome production in South Africa: four case studies. Metallurgical and Materials Transactions B, vol. 46, no. 5, pp. 1-11. [ Links ]
Niemelä, P., Krogerus, H., and Oikarinen, P. 2004. Formation, characterisation and utilisation of CO-gas formed in ferrochrome smelting. Proceedings of the 10th International Ferroalloys Congress, Cape Town, South Africa, 1-4 February 2004. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 68-77. [ Links ]
Peter, D., Andreas, E., Michael, K., and Dirk, M. 2010. Recent development in slag treatment and dust recycling. Steel Research International, vol. 80, no. 10. pp. 737-745. [ Links ]
Pillay, K., Von, B.H., and Petersen, J. 2003. Ageing of chromium(III)-bearing slag and its relation to the atmospheric oxidation of solid chromium(III)-oxide in the presence of calcium oxide. Chemosphere, vol. 52, no. 10. pp. 1771-1779. [ Links ]
Samada, Y., Miki, T., and Hino, M. 2011. Prevention of chromium elution from stainless steel slag into seawater. ISIJ International, vol. 51, no. 5. pp. 728-732. [ Links ]
Song, S. and Garbers-Craig, A.M. 2016. Formation, leachability and encapsulation of hexavalent chromium in the Al2O3-CaO-Fe2O3-Cr2O3 system. Journal of the European Ceramic Society, vol. 36, no. 6. pp. 1479-1485. [ Links ]
Tae, S.J. and Morita, K. 2017. Immobilization of Cr (VI) in stainless steel slag and Cd, As and Pb in wastewater using blast furnace slag via a hydrothermal treatment. Metals and Materials International, vol. 23, no. 3. pp. 576-581. [ Links ]
Zhong, L., Yang, J., Liu, L., and Xing, B. 2015. Oxidation of Cr(III) on birnessite surfaces: The effect of goethite and kaolinite. Journal of Environmental Sciences, vol. 37, no. 11. pp. 8-14. [ Links ],
Paper received Mar. 2017
Revised paper received Sep. 2017














