Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.118 n.5 Johannesburg May. 2018
http://dx.doi.org/10.17159/2411-9717/2018/v118n5a2
STUDENT PAPERS
Test work to examine the potential for improving gold leaching performance at Navachab Gold Mine, Namibia
M.N. Amwele; D.R. Groot
Department of Mining and Process Engineering, Namibia University of Science and Technology, Namibia
SYNOPSIS
The Navachab Gold Mine is an open-pit gold operation located in Namibia. The metallurgical flow sheet consists of crushing, milling, leaching by CIP (carbon in pulp), and electrowinning. Extraction in the leach section is approximately 67%, increases to approximately 86% by the end of carbon adsorption, indicating that extraction in the leach section is far from complete. The purpose of this study was to examine the potential for improving the performance of the leach section. Bottle roll leach tests were conducted using different parameters to observe their impact on gold extraction and cyanide consumption. The factors varied included the cyanide concentration and pH. The effect of introducing lead nitrate on the leach performance was also investigated. Furthermore, it is suspected that constituents in the ore that adsorb gold from solution (known as preg-borrowing constituents) suppress the overall extraction of gold, and thus the benefit of CIL (carbon in leach) was investigated. Finally, bottle roll tests as well as plant tests were conducted to determine the influence of residence time, as it was thought that this may be a limiting factor. An increase in extraction was observed when the cyanide levels were increased and when lead nitrate was introduced. The CIL test also showed an improvement in extraction compared to the normal leach tests. An increment in the pH did not show the same benefit, however. Plant tests showed a decline in the solid residue grade with an increase in residence time. The results indicate possible areas for further research in order to improve leach performance.
Keywords: gold leaching, CIP, CIL, cyanidation, lead nitrate, optimization.
Introduction
The Navachab Gold Mine is an open-pit operation that started production in 1989 at an average gold grade of 2 g/t. Ore mineralization includes pyrrhotite, chalcopyrite, arsenopyrite, sphalerite, molybdenite, scheelite, and uraninite (Nortemann et al., 2000). The metallurgical flow sheet comprises crushing, milling, carbon-in-pulp leaching, and electrowinning.
Figure 1 shows the layout of the leach section, which consists of one pre-oxidation tank and eight leach tanks with a total residence time of approximately 18 hours. The throughput is 200 t/h with the feed at 75% passing 75 μm and pulp density 50% solids. Oxygen is injected into the tanks to obtain DO (dissolved oxygen) levels of approximately 25 ppm, while the cyanide concentration is maintained at approximately 200 ppm. The pH ranges between 9.6 and 10.2.
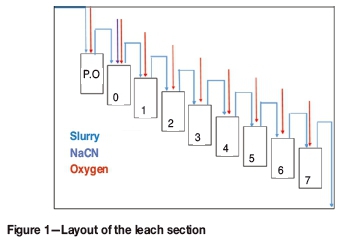
The plant currently achieves an extraction of approximately 67% in the leach section and a final extraction of 86% after adsorption. It is undesirable for leaching to be far from completion before the slurry contacts the carbon because firstly, oxygen as well as cyanide is limited, which limits the amount of gold that actually goes into solution. Secondly, carbon flows countercurrent to the slurry, thus gold losses are likely to occur (especially in the last tank) as the carbon will be pumped upstream without accessing the rest of the soluble gold. The unsatisfactory extraction in the leach section could be attributed to:
► The presence of sulphides like pyrrhotite and arsenopyrite, which can consume cyanide as well as oxygen during leaching.
► Preg-borrowing constituents in the ore, which results in a high content of gold in the leach residue, which is subsequently desorbed from the preg-borrowing constituent by competitive adsorption onto the more active activated carbon in CIP.
► Insufficient residence time in the leach section for the current throughput.
The aim of this research was therefore to investigate whether it is possible to improve the leaching performance, by investigating the following:
► Whether varying certain leach parameters can improve the extraction
► The impact of adding lead nitrate on gold extraction and cyanide consumption
► The possible benefits of CIL (carbon in leach)
► The impact of extending the residence time.
Experimental
Factorial design tests
The three factors investigated were cyanide concentration, pH, and lead addition (as lead nitrate). In order to allow all combinations of factors and levels to be incorporated, a full factorial design was generated using Minitab (2010). Table I shows the different factors and the levels at which they were investigated. Each run was replicated.
The leach tests were carried out using the standard bottle roll method. A slurry sample was collected from the leach feed distribution box. The sample was then filtered and dried. Tap water was added to achieve the desired pulp density of 50% solids. Sodium hydroxide was used for pH modification and 99.5% pure oxygen was bubbled into the slurry. The dissolved oxygen (DO) levels were maintained above 8 mg/l for the duration of each test. Lead nitrate, when used, was added during the first hour. A 20% NaCN (sodium cyanide) solution was then added to achieve the desired concentration and the bottle was rolled for 48 hours. The pH was monitored regularly and adjusted with sodium hydroxide. Samples were taken at 2-hour intervals and filtered, and the filtrate and residue were submitted to the Navachab laboratory for analysis. All samples were analysed for gold to determine the metal extraction. The filtrate was also analysed for final cyanide concentration by titration with silver nitrate.
CIL tests
The objective of this test was to observe the effect of adding carbon earlier during leaching. This test was carried out using the standard bottle roll procedure as outlined above. Table II shows the parameters for the CIL test.
Extended residence time tests
The objective of this test was to observe the effect of extending the residence time on the extraction. A sample of 4 litres was collected from tank 7 (the last tank in the leach train) and subjected to further leaching using bottle roll tests. Table III shows the parameters for the extended residence time test. The test was duplicated.
Monitoring of batch reactor
The Navachab team was also interested in investigating how an extended residence time would affect the extraction on a plant scale. Tank 7 was bypassed and allowed to run in batch mode for 24 hours. The conditions in the reactor mimicked those of the plant in terms of pH, cyanide and dissolved oxygen concentration, and slurry density. Two runs of this test were conducted. Samples were taken at predetermined time intervals. The filtrate and solids were analysed to determine the gold extraction as well as cyanide concentrations. Table IV shows the parameters for the extended residence time test.
Results and discussion
Factorial design tests
It can be observed from Figure 2 that an increase in the cyanide concentration from 200 ppm to 400 ppm resulted in a 0.5% increase in extraction. An increase in cyanide concentration accelerates leach kinetics.
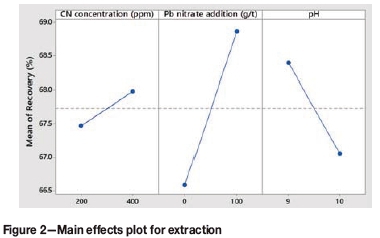
According to Miguel (2014), sulphide ions adsorb strongly onto the gold surface, which suppresses gold leaching. The introduction of lead nitrate resulted in a 2.28% increase in extraction. Studies have shown that the addition of lead nitrate can be beneficial to gold leaching kinetics.
Lead passivates the surface of reactive sulphides and forms insoluble lead sulphide, thus effectively preventing the solution from contacting the sulphides. Lead also decreases complexation with cyanide, thereby reducing cyanide consumption.
An increase in the pH range from 9.6-10.2 to 10.5-11 resulted in a 1.34% decrease in extraction. The electrochemical driving force for gold dissolution is maximized at pH values between 9.0 and 9.5 (Marsden and House, 2006). Thus, it can be inferred that the leach kinetics were likely faster at lower pH values of 9.6-10.2.
Figure 3 shows the main effect of each factor on the cyanide consumption. An increase in the cyanide concentration from 200 ppm to 400 ppm resulted in a 23 g/t increase in cyanide consumption.
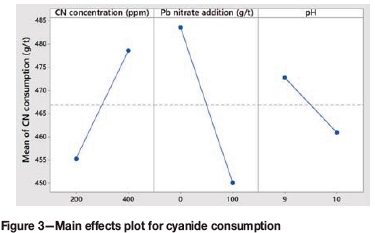
The introduction of lead nitrate resulted in a 34 g/t decrease in cyanide consumption. According to La Brooy, Linge, and Walker (1994), reactions involving sulphides may consume cyanide and oxygen and produce various solution species that can decrease the efficiency of gold leaching. Pyrrhotite is the most reactive iron sulphide mineral in cyanide:
Pyrrhotite >>> Marcasite > Arsenopyrite > Pyrite
The addition of lead nitrate could have resulted in the passivation of these cyanide consumers, resulting in a decrease in the amount of cyanide consumed.
An increase in the pH range from 9.6-10.2 to 10.5-11 resulted in an 11.9 g/t decrease in cyanide consumption. This is probably due to the fact that less cyanide is lost as hydrogen cyanide gas at higher pH values. At a pH of around 9.3, half of the total cyanide is available as HCN (hydrogen cyanide) and half as free cyanide ions. Above pH 10, more than 90% of the total cyanide is available as free cyanide ions, while at a pH of around 8.4 more than 90% of the total cyanide is present as hydrogen cyanide gas. In order to reduce cyanide loss as HCN, cyanide leaching is usually conducted at a pH between 10 and 11 (Marsden and House, 2006).
Higher order interactions were calculated using Minitab (2010) and found to be minimal, and are thus neglected.
CIL tests
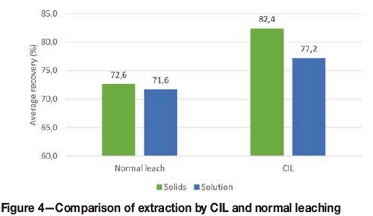
Figure 4 shows that the CIL test achieved the highest average extractions based on both solids and solution analysis. Carbonaceous components in the ore can adsorb dissolved gold during leaching, thus reducing gold extraction. It has been reported that in some cases, as little as 0.1% carbon can have preg-robbing (removing gold from solution irreversibly) or preg-borrowing (removing gold from solution reversibly) properties (Marsden and House, 2006). The higher extractions by CIL are an indication of possible preg-borrowing during the normal leach, which is eliminated by the addition of activated carbon during leaching, thus indicating the benefit of CIL.
Extended residence time and batch reactor tests
Extending the residence time by 4 hours resulted in a significant decrease in the solid residue grade, from 0.66 g/t to 0.43 g/t. This benefit is also confirmed by the extraction curves (Figure 5), which show an increase in the extraction of more than 5% with the longer residence.
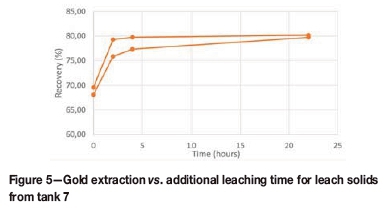
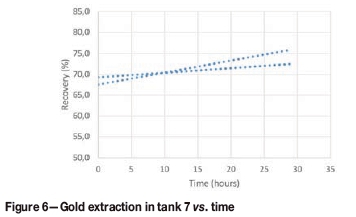
Figure 6 shows the trend lines obtained from the results of the tank 7 test. It can be observed that the extraction increases with time, which shows the potential benefits of adding an extra tank to the leach circuit. The retention time determines the contact time between gold particles and reagents. Typical residence times in a leach section may range from 12-48 hours, depending on the head grade and nature of the ore (Marsden and House, 2006). In practice, most gold plants tend to operate between 24-36 hours' residence time.
Cost analysis
Table V shows the cost analysis that was done for the month of July 2017. The gold price used is that for 17 August 2017.
An increase in cyanide dosage resulted in a 0.5% increase in gold extraction and a 23 g/t increase in cyanide consumption. Adding lead nitrate to leaching increased the extraction by 2.3% and decreased the cyanide consumption by 34 g/t. The lead nitrate consumption was 100 g/t. Increasing the pH decreased the extraction by 1.3% and decreased cyanide consumption by 12 g/t. A higher sodium hydroxide addition was necessary to maintain the higher pH levels.
The cost benefit of changing each parameter was calculated and evaluated as a percentage of the revenue that Navachab achieved for the month of July. The cost analysis showed that an increase in the cyanide concentration and the introduction of lead nitrate can have potential financial benefits. However, an increase in the pH value resulted in a negative net benefit.
Conclusions
An increase in the cyanide levels improves gold extraction but results in increased cyanide consumption. However, the cost analysis showed an overall benefit of increasing the cyanide levels. The addition of lead nitrate improves extraction and reduces cyanide consumption. The introduction of lead nitrate could be very advantageous to Navachab's leaching circuit. An increase in pH reduces cyanide consumption but also reduces gold extraction. This resulted in a negative net benefit.
CIL shows potential benefits in terms of extraction. The ore might contain preg-borrowing matter to the point where it is limiting the extraction of gold. On the other hand, extending the residence time showed similar results to the CIL tests. Thus adding an extra tank to the circuit might be more beneficial to the process than choosing a CIL route. Finally, the results indicate that there is possibly leaching taking place after 18 hours of residence time.
The following measures are recommended.
► Increase cyanide levels but monitor the amount of cyanide lost to the tailings to prevent any environmental issues.
► Plant trials should be performed to verify the benefit of lead nitrate addition. Navachab can decide whether the lead nitrate will be purchased in solution form or as dry crystals. If it is bought in solid form, then a make-up facility will be required. The lead nitrate can be dosed before or during leaching. According to Miguel (2014), these trials can last up to 3 months, and the benefits should be observable after a month.
► Maintain current pH levels.
► Conduct more trials on tank 7, as only two runs were conducted during the course of this research.
► Further test work should be conducted on the benefits of CIL and increasing the residence time.
Acknowledgements
The authors wish to acknowledge Navachab Gold Mine for facilitating this project and the Navachab laboratory team for working tirelessly to analyse all the samples on time. The guidance of Mr Hildebrand Wilhelm, Mr Innocent Matsika, Mr Georg von Oppen, Mr. Jakobus Apollus. and the staff of the Department of Mining and Process Engineering, Namibia University of Science and Technology, is greatly appreciated.
References
La Brooy, S.R., Linge, H.G., and Walker, G.S. 1994. Review of gold extraction from ores. Minerals Engineering, vol. 7, no. 10. pp. 1213-1241. [ Links ]
Marsden, J.O. and House, C.I. 2006. The Chemistry of Gold Extraction. 2nd edn. Society for Mining, Metallurgy & Exploration, Littleton, CO. [ Links ]
Miguel, B. 2014. Lead into gold- A review of the use of lead nitrate to extract more gold. Proceedings of ALTA 2014 Gold-Precious Metals Sessions, Perth, Australia, 29-30 May 2014. ALTA Metallurgical Services. pp. 139-150. [ Links ]
Minitab, 2010. Minitab 17 Statistical Software. State College: Minitab, Inc. [ Links ]
Nortemann, M., Mucke, A., Weber, K., and Meinert, L. 2000. Mineralogy of the Navachab skarn deposit, Namibia: an unusual Au-bearing skarn in highgrade metamorphic rocks. Communications of the Geological Survey of Namibia, vol. 12. pp. 169-177. [ Links ]
Paper received Apr. 2018
Paper written on project work carried out in partial fulfilment of B.Eng (Metallurgical Engineering) degree














