Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 n.11 Johannesburg Nov. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n11a2
SULPHURIC ACID CONFERENCE
Economical abatement of high-strength SO2 off-gas from a smelter
R. Dijkstra; B. Senyard; U. Shah; H. Lee
Chemetics In. Canada
SYNOPSIS
With increasing use of oxygen enrichment and advances in smelting technology, SO2 concentrations in smelter off-gases are increasing, which necessitates larger acid plant equipment and increases in capital and operating. To counteract the shortcomings in conventional acid plants, Chemetics provides two unique solutions: the Chemetics High Strength (CHS™) process and the Chemetics Pseudo-Isothermal process utilizing the CORE™ reactor technology. In this paper we present a general outline of these two solutions and how they can be implemented in new or existing acid plants.
Keywords: smelter off-gas, high-strength SO2, pseudo-isothermal process.
Introduction
Most non-ferrous smelter operations are coupled with a metallurgical sulphuric acid plant to treat SO2-containing off-gases before discharge to the atmosphere. With the increasing use of oxygen enrichment to increase production in existing smelters, and due to advances in smelting technology, more and more smelting operations are producing off-gases with SO2 concentrations well above 30 vol%. The concentrated gases may be mixed with lower concentration off-gases from secondary processing or other emission sources before entering the off-gas cleaning and acid plant.
As smelter off-gases are generally deficient in oxygen, it is necessary to add oxygen, typically using ambient air, to the gas prior to the drying tower in the acid plant. The resulting SO2 concentration in the process gas after this O2:SO2 ratio adjustment is typically between 15 and 25 vol% SO2 at the acid plant converter. However, the conventional sulphuric acid plant (double contact - double absorption) is limited to no more than 13 vol% SO2 at the converter inlet in order to keep the gas temperature leaving the first catalytic stage below the thermal stability limit (approx. 630°C) of the vanadium-based catalyst.
Air addition in excess of the amount required for adjusting the oxygen content increases the gas volume processed through the acid plant and consequently the equipment size, and capital and operating costs. Higher gas throughput also increases the heat loss to the acid circuit, thereby reducing energy recovery from the gas contact section and increasing cooling water demand. With newly built and future smelter operations designed to produce high-concentration SO2 gas at increasing throughputs, ever more air dilution is required to keep within the limits of conventional acid plants. In some cases, gas volumes exceed the design limit of single-train acid plants (currently around 5000 t/d), which forces designers to resort to multiple train contact plants, thus further increasing plant footprint and CAPEX/OPEX.
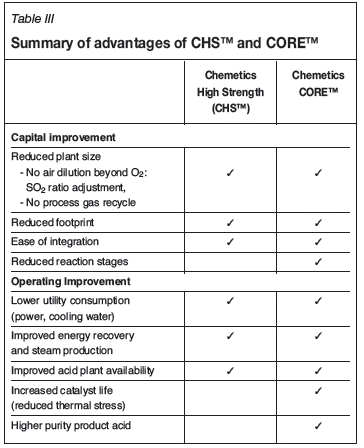
To counteract these shortcomings of the conventional acid plant, Chemetics Inc., with more than 50 years of experience in sulphuric acid technology, now offers two solutions: The Chemetics High Strength (CHS™) process and the Chemetics Pseudo-Isothermal process utilizing the CORE™ reactor technology.
Chemetics High Strength (CHS™) process
The CHS™ process is designed for two typical situations:
> Locations where the gas received from the smelter is high in SO2 but deficient in oxygen, such that dilution air or oxygen is required to achieve the required O2:SO2 ratio for the conversion
> Locations with multiple SO2 off-gas sources (such as a flash furnace coupled with Peirce-Smith converters) that have different SO2 concentrations but are not necessarily deficient in oxygen when mixed together, and where the client is considering installing separate gas-cleaning systems for these gas sources for operational (reliability) or process (e.g. different gas cleaning requirements) reasons.
The CHS™ process capitalizes on the difference in the SO2 concentrations of the feed streams, resulting in the ability to process gases containing up to approximately 18% SO2.
Process description
The CHS™ design (Figure 1) processes the two gas streams (high and low SO2 concentrations) by reconfiguring the contact section. A separate drying tower (with a common acid system) and blower is used for each stream. After drying, the weak gas (which also includes all required dilution air to maintain the correct O2:SO2 ratio) is mixed with part of the strong gas to provide a gas containing approximately 13% SO2 at the inlet of the first catalyst bed. After part of the SO2 is converted to SO3, the now SO3-rich gas from bed 1 is combined with the remaining strong SO2 gas and processed in a further four catalyst beds. The overall arrangement is a 4+1 DCDA configuration. Energy recovery from the hot gas leaving beds 3, 4, and 5 enables the production of high-pressure steam.
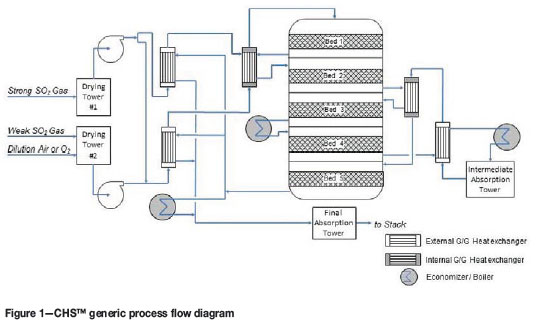
Taking full advantage of Chemetics' experience and expertise in acid plant equipment design, the converter used for the CHS™ process is a single stainless-steel five-bed converter with two internal heat exchangers (see Figure 2). The advantages of the Chemetics stainless steel converter design are well known and include all-welded construction, rapid heat-up time, improved reliability, excellent gas distribution, and less external hot gas ducting.

CHS™ vs. conventional plant - case study
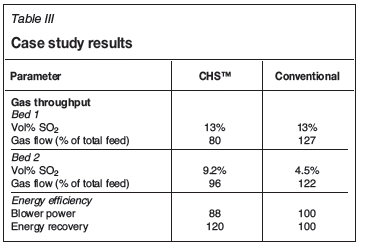
For comparison between the CHS™ and a conventional acid plant, the off-gas sources from a recent study are considered (Table I).
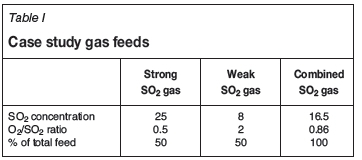
In a conventional acid plant, these sources would be blended and delivered to the drying tower as a single feed stream. Additional dilution air, required to control the first-pass converter bed temperature, results in about 27% increase in the gas throughput. The CHS™ design receives these streams separately and requires no further dilution air. Gas flow through any of the CHS™ converter passes is no more than the total feed gas flow rate. This flow reduction, effectively 25% lower in the study case, translates to capex and opex savings in addition to approximately 20% improvement in energy recovery.
Compared with competitor technology, which recycles hot SO3-rich gas from bed 3 to suppress the temperature rise in the first pass, the CHSTM design offers many benefits (Table II). These include a significantly lower gas flow rate through the converter beds and the heat exchangers upstream of the intermediate absorption tower, higher equilibrium conversion prior to intermediate absorption, reduced power consumption, and fewer reliability concerns associated with a hot SO3 gas recycle fan.
Pseudo-isothermal converter - Chemetics Core™
In August 2016 Chemetics acquired all patents and know-how for the BAYQIK® converter technology from Bayer AG. This converter technology, now marketed under the CORE™ name, is a proven pseudo-isothermal reactor system capable of converting high-strength SO2 gas without diluting the gas with air or recycled process gas. The first commercial installation in Germany (see Figure 3) has been operating continuously for more than 8 years, processing gas with up to 21 vol% SO2. A second plant was commissioned in January 2017 and is processing gas up to 15 vol% SO2. The technology is most valuable in treating a single strong gas source, but can also be an economical pre-converter for a plant with multiple off-gas sources.

Process description
The Chemetics CORE™ converter is the only commercially available isothermal converter system for SO2 oxidation. Continuous removal of the reaction heat using air or molten salt allows the process temperature to be controlled within the operating limit of the catalyst.
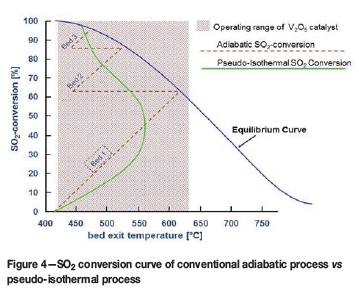
In addition to the ability to convert high-concentration SO2 gas, the pseudo-isothermal process also operates farther from the equilibrium curve than the conventional multi-pass adiabatic process, as shown in Figure 4. This translates into lower overall catalyst loading and significantly higher conversion in a single pass.
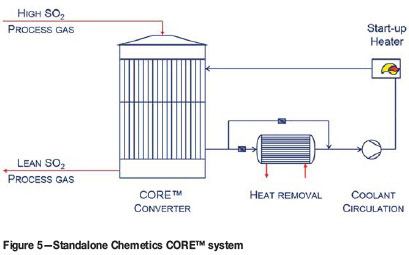
The pseudo-isothermal process is carried out in a patented tubular converter (see Figure 5). The SO2 process gas flows through the tubes, which are filled with a carefully selected mixture of vanadium-based catalyst. A cooling medium (air or molten salt, depending on the reactor size) is introduced on the shell side to remove the reaction heat. Heat transfer is optimized in the design of the reactor. Energy recovered from the circulating cooling medium can be used for preheating the process gas and for generating high-pressure steam. CORE™ reactors designs for capacities up to 100 000 Nm3/h (equivalent to approx. 2000 t/d acid production) are currently available, with higher capacity designs under development.
There are several approaches to using the Chemetics CORE™ technology in handling high-strength SO2 gas.
In-line Chemetics CORE™
In an in-line configuration, the CORE™ reactor can simply replace the primary contact plant, which typically includes beds 1 through 3 and intercooling gas exchangers in an adiabatic design. As a result, the in-line Chemetics CORE™ design reduces not only the gas flow through the plant but also the number of major equipment items and the overall plant pressure drop.
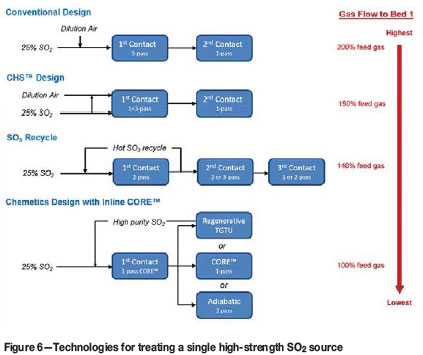
This significant reduction in plant size is demonstrated in the following comparison between the various technologies using a baseline case of 25 vol% SO2 off-gas with adequate oxygen content (O2/SO2 ratio > 0.8). In a conventional design, air addition required to reduce the SO2 concentration to 13 vol% results in near-doubling of the gas flow through the contact plant. The CHS™ design can reduce the dilution air requirement by adjusting about half of the feed gas to 13 vol% prior to bed 1. The resulting gas flow through the acid plant is reduced to 75% of that in the conventional design, but is still elevated at 150% of the feed gas flow. If the hot SO3 recycle approach is used, the gas flow rate to bed 1 will be reduced to about 140% of feed gas flow (or approximately 70% of the gas flow for a conventional design). However, in the absence of dilution air, the number of adiabatic passes must increase to achieve the same SO2 emission allowance. This results in a contact plant with up to six passes and three absorption towers. Finally with the Chemetics in-line design using a CORE™ reactor coupled with secondary contact, the gas flow to bed 1 is not only the lowest but also the number of reaction stages is minimized (see Figure 6).
Since SO2 conversion in excess of 90% can be achieved using a single Chemetics CORE™ reactor, the downstream secondary contact section can be customized based on the client's specific needs. This second SO2 abatement process can be (i) a conventional single or dual adiabatic design, (ii) another single-pass CORE™ reactor, or (iii) a regenerative SO2 tail-gas treatment unit. For instance, if a client desires the smallest plant footprint and the flexibility of equipment modularization, a Chemetics CORE™ reactor coupled with a regenerative tail-gas unit would be the preferred solution, at the expense of steam consumption in the tail-gas unit. This combination is especially suitable for smaller capacities or locations where a regenerative scrubbing system is already required to capture the SO2 gases prior to conversion to acid.
Chemetics CORE™pre-converter
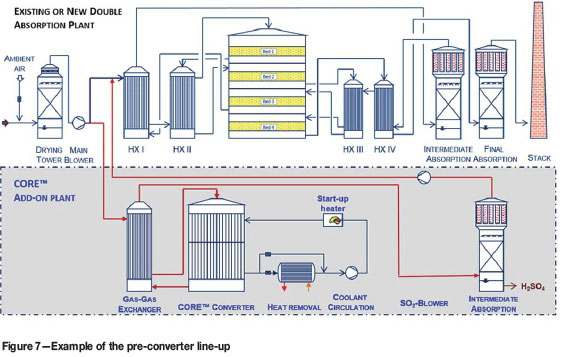
Another application of the technology is to treat only a portion of the strong SO2 feed gas, with the SO3-rich gas leaving the isothermal pre-converter directly mixed with rest of the strong feed gas and processed through a standard adiabatic contact plant. This configuration may be attractive for large-capacity new plants, where the benefits of directly treating a strong feed gas are fully realized with a smaller CORE™ reactor and cooling system. This same concept also highlights the value of the reactor technology for brownfield plant expansion, where a planned upgrade in the smelter operation would increase the SO2 gas concentration going to an existing acid plant. The conventional design would require diluting the strong gas with air, resulting in a gas flow rate beyond the capacity of the existing acid plant. In many cases, a new double-contact acid plant or a costly debottlenecking of the existing contact plant would be required. In such applications, an add-on Chemetics CORE™ module is a more economical solution. The CORE™ module converts and removes the extra SO2, resulting in a gas to the existing acid plant that is the same volume and concentration as before the smelter expansion. This solution thus offers a compact design, smaller footprint, and improved energy efficiency at lower capital and operating cost.
An example of the pre-converter line-up is shown in Figure 7. Optional add-ons such as a dedicated SO2 booster fan and intermediate absorption tower are offered, depending on the project requirement. Acid produced in the intermediate absorption tower is of high quality as any remaining impurities in the process gas have already been washed out in the drying tower. In some cases this premium quality acid can be sold at significantly higher prices, improving profitability.
The Chemetics CORE™ converter operation can be adjusted by controlling the temperature of the cooling medium (controlling conversion) or by adjusting the gas flow through the reactor. If the SO2 concentration from the smelter is low, the unit can be taken off-line into 'hot standby' and can stay in this mode for any length of time while maintaining optimum catalyst temperature for immediate restart. From hot-standby mode the plant can be switched to on-line by simply restarting the gas flow. This operational flexibility maintains a steady gas concentration to the downstream acid plant despite variability in feed gas, and thereby improves the acid plant reliability.
The versatility of the Chemetics CORE™ technology, with its ability to handle high-strength SO2 gas and fluctuating process conditions, makes it a powerful solution for metallurgical SO2 off-gas abatement which also allows for increased steam production. When used in smelter expansions to accept higher concentration gas, the CORE™ technology is by far the most cost-effective solution.
Summary and Conclusions
Chemetics offers several solutions for treating high-strength SO2 off-gas without requiring excess dilution air or recycling of hot process gas. While CHS™ is best suited for applications with multiple large SO2 gas streams, process designs integrating the Chemetics CORE™ reactor can directly treat concentrated gas streams as high as 50 vol% SO2. Both approaches use proven equipment and catalyst and simple controls. Their advantages over the conventional acid plant design are summarized in Table III. Chemetics' success in the sulphuric acid industry has been built on focusing on the client's needs. With the evolution of increasingly high-concentration SO2 off-gases, Chemetics is able to offer customized solutions for any conditions by selecting the appropriate process. The optimized solution is reached by closely working together with our clients to ensure that their needs are fully realized.