Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Journal of the Southern African Institute of Mining and Metallurgy
versión On-line ISSN 2411-9717
versión impresa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 no.10 Johannesburg oct. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n10a1
CONFERENCE PAPERS
Reaction kinetics of ZrF4 chloridation at elevated temperatures
N.J.M. GroblerI; C.J. PostmaII; P.L. CrouseI
IFluoro-Materials Group, Department of Chemical Engineering, University of Pretoria, South Africa
IISouth African Nuclear Energy Corporation (Necsa), South Africa
SYNOPSIS
Zirconium metal is used in the alloy cladding of nuclear fuel rods. Necsa produces ZrF4 by reacting ammonium bifluoride with desilicated plasma-dissociated zircon. The ZrF4 then undergoes a sublimation separation step to reduce the Hf content to below 100 ppm Hf. The ZrF4 is converted into ZrCl4via its reaction with magnesium chloride. The ZrCl4 is then used in a plasma process to produce Zr metal for use in the nuclear industry. The aim of the research reported here is to obtain a first-order estimate of the reaction kinetics for the chloridation reaction. This was done using the data obtained from a dynamic thermogravimetric analysis experiment and minimising the error between experimentally determined degrees of conversion and predictions of a stepwise kinetic model predictions. The reaction was found to take place only above the sublimation point of ZrCl4. At a low enough heating rate it can be assumed the loss in mass is due to sublimation of ZrCl4, the moment it is formed and the rate of mass loss is equal to the reaction rate.
Keywords: reaction kinetics, zirconium chloride, thermogravimetric analysis.
Introduction
Zirconium is used in the nuclear industry in the cladding of nuclear fuel rods. Zirconium naturally occurs with 1-3 % hafnium; nuclear grade zirconium needs to be purified to contain less than 100 ppm hafnium (Smolik, Jakbik-Kolon and Poraski, 2009). Part of the process developed by Necsa is to convert ZrF4 to ZrCl4 after the sublimation purification step (Postma, Niemand and Crouse, 2015). Makhofane et al. (2013) described a method for the chloridation of ZrF4 according to Reaction [i] below:
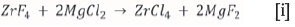
The objective of this research is to estimate the reaction kinetics for the solid state chloridation of ZrF4 sufficient for scale-up and reactor design. The reagents were prepared with a slight molar excess of MgCl2 and dynamic thermogravimetric analysis (TGA) at a very low temperature ramp rate to ensure minimal thermal lag, was used to monitor the reaction.
Experimental
The materials were obtained from Necsa SOC Ltd and were prepared through the method discussed by Nel et al. (2011). Each reagent was carefully weighed with a 10 % molar excess of MgCl2. The reagent mixture was roughly 6.4 mg ZrF4 and 8 mg MgCl2. Aluminum crucibles were used. The reagents were prepared under atmospheric conditions due to the non-availability of a glove box. The MgCl2 thus became partially hydrated due to moisture in the air. The degree of hydration was calculated from the first decomposition step where two H2O molecules are released.
The TGA was programmed to heat at a constant rate of 1 °C per minute from room temperature to 500 °C and remain there for 10 minutes before cooling down. The analysis was done with 200 mL-min-1 N2 as carrier gas. A Hitachi STA 7300 TGA-DTA was used for the work.
The code for data fitting was developed in-house and written in Python. It comprised prediction of the sample-mass as a function of time and temperature by integration of the rate expressions using estimated kinetic parameter values, then minimising the difference between predicted and experimental values by refinement of the parameters.
Results and discussion
Figure 1 shows the dynamic thermogram of the reaction.
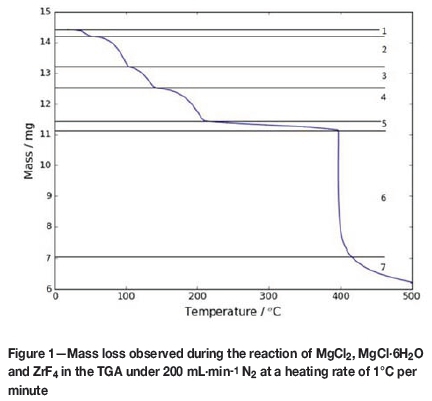
MgCl2 is hygroscopic and hydrates readily under ambient conditions. Since we did not have the equipment to work under anhydrous conditions, we had to work with wet material and included this in our data analysis. Steps 1-5 and step 7 in Figure 1 are accounted for by dehydration, while step 6 is the actual chloridation reaction.
An important assumption for the model is that the magnesium chloride starting material is a mixture of anhydrous MgCl2 and fully hydrated MgCl2-6H2O and that the chloridation is effected by anhydrous MgCl2 rather than the hydrated material, or the oxychloride that forms during the dehydration of the hexahydrate.
MgCl2-6H2O loses crystal water upon heating through several decomposition steps (Kirsh, Yariv and Shoval, 1987; Huang et al., 2010). The dehydration of MgCl2-6H2O is described by Reactions [ii] to [vii] below:
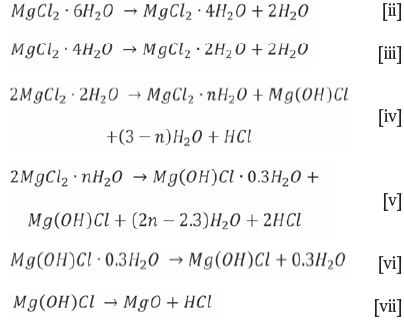
It is important to note that MgO, not MgCl2, is the final product. The formation of gaseous product is reflected on the thermograms as a loss of mass.
Kirsh, Yariv and Shoval (1987) and Huang et al. (2010) derived kinetic data for the thermal decomposition of MgCl2-6H2O. It should be noted that neither was done according to most recent ICTAT specifications (Vyazovkin et al., 2011) and the kinetic triplets reported should be regarded as estimates rather than correct absolutes. In this work we similarly aim at obtaining engineering values, rather than exact kinetics, for the chloridation reaction.
While the focus of this work is the chloridation reaction itself, the dehydration had of necessity to be incorporated to estimate the amount of dry MgCl2 present. The kinetic data given by Huang et al. (2010) was used for ease of implementation. Their kinetic models for the individual steps were adopted without change, while their pre-exponential factors and activation energies were used as starting values for obtaining a fit to our data.
The first five dehydration steps, numbered 1-5 in Figure 1, correspond to Reactions [ii] to [vi] and to Equations [2] to [6] below. The chloridation of ZrF4, which starts at 396°C, is marked as step 6, corresponding to Reaction [i] and to Equation [1]. This is followed by a further loss of HCl as the hydroxide chloride, marked as step 7 and corresponding to Reaction [vii] and Equation [7].
The fitting procedure involved minimising the error between the model predictions and the experimental data, by adjusting the pre-exponential constant in the kinetic equations for the six decomposition steps and in the chloridation reaction itself, as well as the exponent in Equation [1].
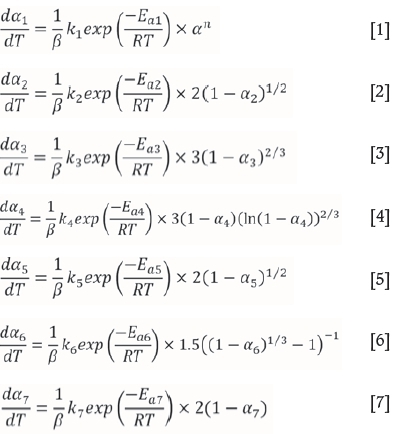
where m0 and mf are the initial and final masses, ß is the heating rate, ki the pre-exponential factors and Eai the activation energies.

where m0 and m f are the initial and final masses, ß is the heating rate, kithe pre-exponential factors and Eai the activation energies.
The form of the dehydration equations given by Huang et al. (2010) can be attributed to the solid-state process mechanism used to fit the decomposition steps. The mechanisms that resulted in the best fit for each step are: two-dimensional phase boundary mechanism, three-dimensional phase boundary mechanism, nucleation and nuclei growth mechanism (Avrami-Erofeev equation n=3), two-dimensional phase boundary mechanism, three-dimensional diffusion mechanism (cylinder and G-B equation) and nucleation and nuclei growth mechanism (Avrami-Erofeev equation n=1), respectively. The general form of the kinetic data given by Huang et al. (2010) is shown in Equation [9]:

wheref(α) is termed the kinetic model, for a single step and is given by the above-mentioned mechanisms for solid-state processes. For the chloridation reaction we assumed the simple empirical model,f (a)= αn.
Table I shows the values of the pre-exponential constant, the activation energy and the temperature ranges during which each stage is active.
The value of n, the exponent in the kinetic model for the chloridation step, Reaction [i], stage 6 was found to be 1.5. Figure 2 shows the experimental data along with the fitted kinetic model. The Euclidian norm of the difference between the experimental data and model prediction was 0.018, indicating a good approximation.
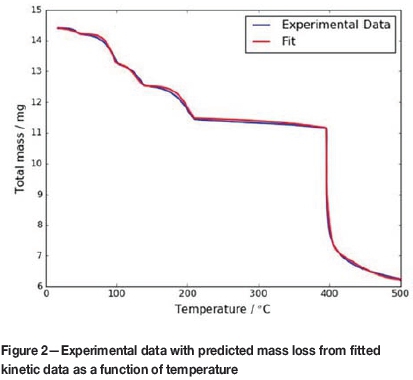
In the model each a was assigned a range 0-1. The initial and final masses of each were estimated from the thermogram. These mass values correspond to the temperature ranges for each step listed in Table I.
The thermodynamically computed vapour pressure of zirconium tetrachloride is given in Figure 3. The inflection point of the sublimation curve is at 327°C.
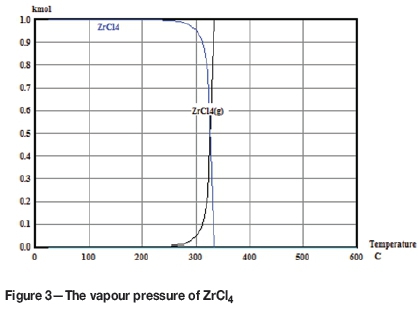
The onset temperature for the chloridation process, step 6, is 396°C. This suggests that the reaction is more complex than a typical solid-solid reaction where contact and mass transfer are problematic, causing very slow reaction rates. The chloridation takes place rapidly at a temperature where gaseous zirconium tetrachloride is the thermodynamically preferred phase and this reaction product can be released as soon as it is formed.
Conclusions and recommendations
The work presented here yielded fair kinetic data, sufficient for reactor and process design. It further addresses the very real prospect that industrially it may be extremely difficult to use pure anhydrous MgCl2 as reagent, due to its hygroscopic nature. In order to make the research scientifically more rigorous, a number of issues need to be addressed in future work. The most important of these are:
► Experimental work under isothermal conditions are required for exact kinetic data
► Anhydrous kinetics has to be decoupled from the kinetics of the reaction of ZrF4 with the hydrated species, using pure compounds
► The exact hydrated magnesium species formed have to be determined as function of degree of bulk hydration
► Physical examination of the product at various stages during the reaction has to be done, by e.g. electron microscopy, to determine the mass transfer mechanisms that apply
The kinetic triplets for the dehydration steps, reported in the literature and used here, need to be revisited.
The pre-exponential factors and activation energies are unrealistically high in general and more extensive experimental work is required to achieve physically realistic values.
References
Huang, Q.Z., Lu, G.M., Wang, J. and Yu, J.G. 2010. Mechanism and kinetics of thermal decomposition of MgCl2.6H2O. Metallurgical and Materials Transactions B, vol. 41, no. 5. pp. 1059-1066. [ Links ]
Kirsh, Y., Yariv, S. and Shoval, S. 1987. Kinetic analysis of thermal dehydration and hydrolysis of MgCl26H2O by DTA and TG. Journal of Thermal Analysis, vol. 32, no. 2. pp. 393-408. [ Links ]
Makhofane, M.M., Nel, J.T., Havenga, J.L. and Afolabi, A.S. 2013. Chlorination of anhydrous zirconium tetrafluoride with magnesium chloride. Proceedings of the Precious Metal Development Network Conference, Cape Town, South Africa, 14-16 October 2013. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 339-348 [ Links ]
Nel, J.T., du Plessis, W., Crouse, P.L. and Retief, W.L. 2011. Treatment of zirconia-based material with ammonium bi-fluoride. US patent 8778291 B2. South African Nuclear Energy Corporation Limited. [ Links ]
Postma, C.J., Niemand, H.F. and Crouse, P.L. 2015. A theoretical approach to the sublimation separation of zirconium and hafnium in the tetrafluoride form. Journal of the Southern African Institute of Mining and Metallurgy, vol. 115, no. 10. pp. 961-965. [ Links ]
Smolik, M., Jakbik-Kolon, A. and Poraski, M. 2009. Separation of zirconium and hafnium using diphonix chelating ion-exchange resin. Hydrometallurgy, 95 3-4, pp. 350-353. [ Links ]
Vyazovkin, S., Burnhamb, A.K., Criadoc, J.M., Pérez-Maquedac, L.A., Popescud, C. and Sbirrazzuolie, N. 2011. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochimica Acta, vol. 520, nos. 1-2. pp. 1-19. [ Links ] ♦
This paper was first presented at the AMI Precious Metals 2017 Conference 'The Precious Metals Development Network' 17-20 October 2017, Protea Hotel Ranch Resort, Polokwane, South Africa.














