Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 no.8 Johannesburg Ago. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n8a1
HYDROMETALLURGY CONFERENCE 2016
Physical and chemical transformations of gangue materials during leaching of copper sulphides, and their influence on copper leaching kinetics
T. Vargas; F. Rojas; C. Bahamondez; R. Castro; C.F. Ihle; M. Caraballo; E. Widzyk-Capehart
Advanced Mining Technology Center (AMTC), University of Chile, Santiago, Chile
SYNOPSIS
This work reports preliminary results from investigations of the chemical and physical transformations of gangue materials during ferric leaching of a copper sulphide ore in sulphuric acid solutions, and their effect on copper leachability. Experiments were conducted with sulphide ore containing 0.6% Cu, consisting mainly of chalcopyrite, chalcocite, and covellite particles in the size range -3/8 +1/4 inches (6.3-9.5 mm). Leaching was conducted on small flooded ore beds using solutions containing 10 g/L H2SO4 and 3 g/L Fe3+. Leaching solutions were maintained at a constant pH (pH = 1) and at a constant Eh (0.75 V/NHE) during the experiments. Leaching progress was monitored through analysis of Cu, Fe, Si, Al, Ca, and Mg in solution. Chemical transformations in the ore were characterized by XRD and changes in the internal porosity of ore particles were characterized by measuring nitrogen adsorption with a sorptometer. The results showed a fivefold increase in internal porous area of the ore particles due to selective leaching of gangue species under acid attack. Variation in ore porosity influences the kinetics of copper dissolution, presumably due to the liberation of sulphide particles occluded in the gangue.
Keywords: ferric leaching, copper sulphide, porosity, gangue minerals.
Introduction
Heap leaching is a well-established technology that enables the economic processing of various types of low-grade ores in the copper industry, which could not otherwise be exploited (Ghorbani et al., 2011; Watling, 2006). In the case of secondary sulphide copper ores, heap leaching can offer a process alternative that is economically more attractive than the conventional concentration and smelting route. Compared to other extractive technologies, heap leaching is unique in that relatively large particles sizes are treated, typically 12-25 mm top size for crushed and agglomerated ores and larger for run-of mine dump leaching operations or in-situ leaching. Accordingly, despite much progress since it was first applied in recent times, the process remains limited by low recoveries and long extraction times.
The rate of copper leaching and the extent of final copper recovery from heaps, dumps, and in situ operations are to a great extent limited by the limited access of leaching solutions to copper sulphide particles occluded in the gangue (Watling et al., 2014). Increased access of the solution to the copper sulphides can be gained by further reducing the ore particle size by crushing, when economically acceptable. In fact, a relationship between the percentage of copper minerals exposed with respect to particle size can provide a good basis for predicting copper recovery for a known particle size distribution (Miller et al., 2003; Dhawan et al., 2012). It would be economically advantageous to find ways of improving the leaching rate of copper minerals that remain occluded in the gangue without further reduction in particle size. The leaching rate is very slow as it depends on the diffusion of lixiviant and soluble products through gangue materials that encapsulate the copper minerals, and which can present very low natural porosity (Watling et al., 2014). Leaching improvements have been obtained by utilizing crushing techniques such as the high-pressure grinding roll (HPGR), which induces a high level of microfracturing that increases diffusion rates in the gangue (Ghorbani et al., 2013). The influence of microfracturing produced by blasting on copper leaching recoveries has been also investigated (Parra et al., 2015).
It is well known that during leaching there is an important degree of gangue dissolution under acid attack (Hiskey, 1992). This is shown by the incorporation of a wide variety of ions into the pregnant leach solution (Crundwell, 2015). However, little research has been done to study the influence that this dissolution process could have on the structure of the gangue and, eventually, on the mechanism of dissolution of copper mineral particles. The present work is part of a research programme aimed at identifying chemical and biological routes to improve the access to and leachability of mineral particles occluded in the ore. Experimental results so far obtained have enabled the quantification of the porosity created by acid attack on gangue materials during ferric leaching of copper sulphide ores, and led to investigations of the influence of acid pretreatment on copper leaching rate.
Experimental
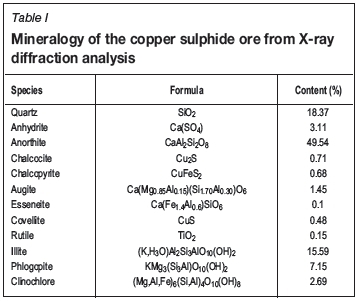
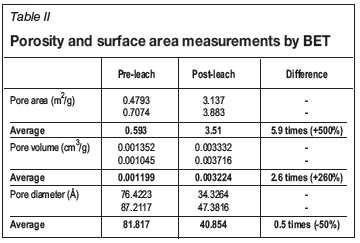
Leaching experiments were conducted on a copper ore sample with particle size in the range -3/8 +1/4 inches (6.3-9.5 mm). The ore contained 0.6% Cu, 6.1% Fe, 5.6% S, 51.8% Si, 21% Al, 6.8% Ca, and 2.5% Mg. Cu was mainly present in chalcopyrite, chalcocite, and covellite. The contents of the main species present in the ore, obtained from X-ray diffraction analysis, are listed in Table I. Leaching experiments were conducted in a system of flooded differential ore columns, which consisted of small ore beds with a mass of about 600 g completely immersed in about 3 L of leaching solution. The solution was gently stirred during the leaching experiment to circulate it inside the ore bed and prevent the formation of internal concentration gradients of reactants (see Figure 1).
Three different experiments were conducted. In experiment A the ore was leached for 288 hours with a solution containing 10 g/L H2SO4 and 3 g/L Fe+3; in experiment B, which was an acid pre-treatment, the ore was leached for 288 hours with a solution containing only 10 g/L of H2SO4; in experiment C the same ore initially leached in Experiment B was leached for a further 264 hours with a solution containing 10 g/L H2SO4 and 3 g/L Fe+3.
Experiments were conducted at 30°C under iso-pH conditions (pH 1), maintained by periodical addition of H2SO4, and under iso-Eh conditions (0.75 V/NHE), maintained by periodical additions of peroxide. Leaching progress was monitored by analysis of Cu, Fe, Al, Ca, Si, and Mg in the leaching solution. Chemical transformations of the ore in experiment A were characterized by XRD analysis of the initial and leached ore samples. Changes of internal porosity of ore particles in experiment A were characterized by analysis of the initial and leached samples with a sorptometer (Micromeritics ASAP 2010), which measured the internal area of the particles by adsorption of nitrogen by the BET method (Brunauer, Emmett, and Teller, 1938).
Results and discussion
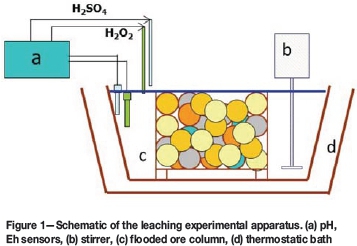
The dissolution results for experiment A, which corresponds to ferric leaching of the ore, are shown in Figure 2. Copper reached a maximum concentration of 27 mg/L in solution, which corresponds to 2.3% recovery. Solution concentrations of elements associated with gangue minerals were 664 mg/L Ca (5.0% recovery), 210 mg/L Mg (4.3% recovery), 223 mg/L Al (0.54 % recovery), and 321 mg/L Si (0.32 % recovery). The presence of gangue elements in solution can in principle be attributed to dissolution of clinochlore, phlogopite, augite, and anhydrite, the contents of which were drastically reduced in the leach residue. However, the percentage of dissolved calcium cannot be explained solely on these grounds, and indicates that a fraction of the anorthite was also dissolved.
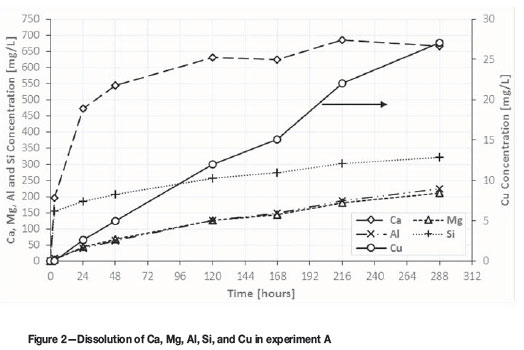
Table II shows the results of porosity measurements of the copper ore before and after leaching in experiment A. The leaching greatly increased the surface area of the ore, from 0.593 to 3.51 m2/g, which represents an increase of about six times. The porosity of the leached residue was 2.6 times greater than in the untreated ore. The average pore diameter in the leached sample, however, was smaller than in the untreated ore sample, and varied from 81.8 to 40.8 Ǻ. This indicates that the newly created porosity consisted of a network of pores much smaller than those originally present in the ore.
The porosity increase observed in experiment A can be in principle related to partial dissolution of gangue minerals under acid attack, as observed from the amounts of Al, Ca, Mg, and Si dissolved. In order to assess the potential effect of the porosity increase on copper leaching, a series of experiments was designed in which copper dissolution was measured during ferric leaching of an ore sample after acid pretreatment. Accordingly, the fresh ore was initially leached in a solution containing only 10 g/L of H2SO4 (experiment B) and immediately thereafter was further leached in a solution containing 3 g/L Fe+3 and 10 g/L H2SO4 (experiment C).
Figure 3 shows the dissolution results for experiment B. Comparison of Figures 2 and 3 shows that the rate of dissolution of Ca, Al, Si, and Mg was very similar in both experiments, confirming that these ions originate from the dissolution of gangue minerals by H2SO4, which was at the same concentration in both experiments. The copper extraction in experiment A (2.32%) was about three times acid leaching is unexpectedly high, considering that the sample did not contain copper oxides. Copper leaching in this case can be explained in part as the result of the oxidative leaching of copper sulphides by ferric ions released during the dissolution of iron-containing gangue species such as clinochlore, and partial oxidation by dissolved oxygen. It is also possible that it originates from acid dissolution of chalcocite.
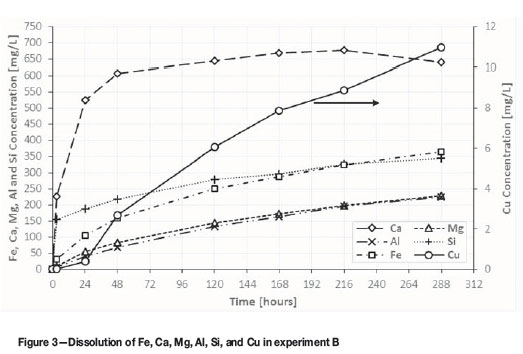
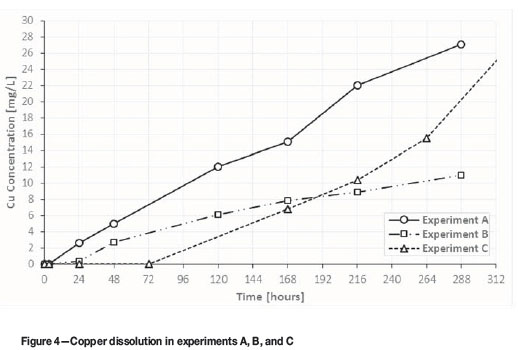
Figure 4 shows the copper dissolution for experiments A, B, and C. Copper dissolution in experiment C started slowly, probably due to the initial depletion of sulphide particles directly exposed on the surfaces of the ore particles, which were presumably leached during the acid pretreatment step. However, after 72 hours of leaching, the copper dissolution rate in experiment C began to increase continually. In fact, after 264 hours of leaching in experiment C, copper dissolution exceeded that obtained in the same leaching time in experiment B, by ferric leaching without acid pretreatment.
It has been reported that in porphyry copper ores, copper sulphides can be associated with quartz, feldspars, and some types of mica (Watling et al., 2014). In the ore used in the current study, it is very likely that an important fraction of the copper sulphides is associated with anorthite, a feldspar that constitutes about 50% of the gangue (Table I). It is well known that feldspars are subject to acid attack during heap leaching, which causes accumulation of potassium, sodium, aluminium, and silica in processing circuits (Crundwell, 2015). Therefore, the increase in copper leaching rate observed here is presumably due to the liberation of copper sulphide particles by partial dissolution of anorthite under acid attack.
Conclusions
Secondary sulphidic copper ores subjected to ferric leaching with 3 g/L Fe+3 in 10 g/L sulphuric acid solutions underwent a dramatic increase in internal porosity. Pretreatment of the ore with acid leaching confirmed that the porosity increase was related to selective dissolution of gangue minerals under acid attack. The increase in gangue porosity influences the rate of copper leaching, suggesting that acid attack of the gangue contributes to liberation of some sulphide particles occluded in the gangue.
Further research is necessary to identify the mechanism of porosity formation under acid attack and quantify its influence on the copper leaching rate.
Acknowledgements
Financial support from Conicyt Basal Project FB0809 is gratefully acknowledged.
References
Brunauer, S., Emmett, P.H., and Teller, E. 1938. Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, vol. 60. pp. 309-319. [ Links ]
Crundwell, F.K. 2015. The mechanism of dissolution of the feldspars: Part I. Dissolution at conditions far from equilibrium. Hydrometallurgy, vol. 151. pp. 151-162 [ Links ]
Dhawan, N., Safarzadeh, M.S., Miller, J.D., Moats, M.S., Rajamani, R.K., and Lin, C-L. 2012. Recent advances in the application of X-ray computed tomography in the analysis of heap leaching systems. Minerals Engineering, vol. 35. pp. 75-86. [ Links ]
Ghorbani, Y., Becker, M., Mainza, A., Franzidis, J-P., and Petersen, J. 2011. Large particle effects in chemical/biochemical heap leach processes - a review. Minerals Engineering, vol. 24. pp. 1172-1184. [ Links ]
Ghorbani, Y., Mainza, A.N., Petersen, J., Becker, M., Franzidis, J-P., and Kalala, J.T. 2013. Investigation of particles with high crack density produced by HPGR and its effect on the redistribution of the particle size fraction in heaps. Minerals Engineering, vol. 43-44. pp. 44-51. [ Links ]
Hiskey, J.B., Oner, G., and Collins, D.W. 1992. Recent trends in copper in situ leaching. Mineral Processing and Extractive Metallurgy Review, vol. 8. pp. 1-16. [ Links ]
Miller, J.D., Lin, C.L., Garcia, C., and Arias, H. 2003. Ultimate recovery in heap leaching operations as established from mineral exposure analysis by X-ray microtomography. International Journal of Mineral Processing, vol. 72. pp. 331-340. [ Links ]
Parra, H., Onederra, I., Michaux, S., Kuhar, L., McFarlane, A., and Chapman, N. 2015. A study of the impact of blast induced conditioning on leaching performance. Minerals Engineering, vol. 74. pp. 1-12. [ Links ]
Watling, H.R. 2006.The bioleaching of sulphide minerals with emphasis on copper sulphides - a review. Hydrometallurgy, vol. 84. pp. 81-108. [ Links ]
Watling, H.R., Shiers, D.W., Li, J., Chapman, N.M., and Douglas, G.B. 2014. Effect of water quality on the leaching of a low-grade copper sulfide ore. Minerals Engineering, vol. 58. pp. 39-51. [ Links ]














