Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 n.7 Johannesburg Jul. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n7a7
PAPERS OF GENERAL INTEREST
Entrained defects in light metal cast alloys
M.A. El-Sayed; M. Ghazy
Arab Academy for Science and Technology and Maritime Transport, Abu Qir, Egypt
SYNOPSIS
The properties of light alloy castings are strongly affected by their inclusion content, particularly double oxide film defects (bifilms), which not only decrease the tensile and fatigue properties, but also increase their scatter. Recent research has suggested that oxide film defects may alter with time, as the air inside the bifilm would react with the surrounding melt. In this work, the effect of time on double oxide film defects is studied for different Al alloys. The results suggest that bifilm defects, once entrained, experience changes in their internal atmospheres which significantly affect their morphology and their influence on the alloy's mechanical properties. These changes involve the consumption of both oxygen and nitrogen inside the defect (with the former occurring first), which enhances the mechanical properties, but this is followed by hydrogen diffusion into the bifilms with a corresponding adverse effect on the properties.
Keywords: double oxide film defects, aluminium, casting, hydrogen.
Introduction
As the use of cast aluminium has increased, so have the mechanical property requirements (El-Sayed, 2015; Youssef and El-Sayed, 2016). Since the mechanical properties of Al castings are greatly affected by their inclusion content, it is important to study these inclusions, their types, causes, and harmful influences. One of the most significant inclusions is the double oxide film defect, which has been reported to have very detrimental effects on the reliability and reproducibility of Al castings (Campbell, 2003).
During the casting of aluminium alloys, the molten surface is exposed to air, which results in the formation of a surface oxide film. As the liquid metal is transferred or poured, its surface can experience disturbances or breaking waves (known as surface turbulence), resulting in the surface of the liquid metal folding over onto itself. This causes the upper and lower oxidized surfaces to come together and trap a layer of the mould atmosphere between them, creating a double oxide film defect or 'bifilm'. This defect can be incorporated into the bulk liquid by entrainment as shown in Figure 1 (Campbell, 2003, 2006).
Entrained double oxide film defects represent the easiest possible initiating sites for cracks, hot tears, or pores, as their unbonded dry inner surfaces can be separated with minimal effort because of the presence of an internal atmosphere. In a study of the fatigue properties of Al-7Si-0.4Mg alloy castings, Nyahumwa, Green, and Campbell (1998a) reported that in about 98% of all fatigue fractures, bifilms were the initiators of cracks. The remaining 2% of samples that did not contain bifilms exhibited up to 100 times greater fatigue lives.
Campbell (2006) suggested that after entrainment and due to internal turbulence in the bulk liquid, the entrained bifilm could become compacted into a convoluted form. Afterwards, the bifilm might unfurl in the quiescent conditions of the mould cavity and then re-establish its shape as a planar crack. Moreover, during solidification, the solubility of hydrogen in the alloy decreases significantly, and it may diffuse into the bifilm gap, causing it to expand into a crack or pore. It has also been shown that double oxide films could be favourable sites for the nucleation and growth of a wide variety of intermetallic compounds (Cao and Campbell, 2003), as shown in Figure 2.
Bifilms, either acting as cracks or helping in the nucleation of porosity or intermetallic phases, can therefore be detrimental to the mechanical properties of Al castings. They not only reduce the elongation, tensile strength, and fatigue limit of the castings, but also increase the variability of these properties (El- Sayed, 2016; El-Sayed and Griffiths, 2014).
Nyahumwa, Green, and Campbell (1998b) suggested that, after entrainment, the oxide layer might transform from y-Al2O3 to a-Al2O3, a process that might take about 5 hours. This transformation is associated with a volume decrease of about 24%, which would create stresses in the oxide skin, resulting in cracking. This would allow the oxygen and nitrogen inside the defect to react with the surrounding melt. With time, all the bifilm atmosphere would be consumed, possibly allowing other processes to operate that might lead to the deactivation of the defect.
Raiszadeh and Griffiths (2006) established a methodology to study the history of oxide films in an Al melt. Their results showed that, because of the higher free energy of formation of Al2O3, the oxygen in the trapped air inside a double oxide film defect would be consumed, first to form Al2O3, then the nitrogen would react to form AIN. These reactions would reduce the volume of the trapped air bubble. Also, if the initial hydrogen content of the melt was higher than the equilibrium associated with the ambient atmosphere, hydrogen would diffuse into the trapped air bubble and increase its volume. The reaction rates of the trapped air within the defect were utilized to build a semi-empirical mathematical model to predict the duration of the atmosphere inside a double oxide film defect. The results suggested that the consumption of oxygen and nitrogen inside the defect would not take more than about three minutes (Raiszadeh and Griffiths, 2008).
The aim of the current work was to study the effect of holding an Al alloy melt in the liquid state on the shape of the entrained oxide film defects, and hence on the mechanical properties of the resulting castings. The main objective was to learn whether oxide film defects could be eliminated, or at least whether their deleterious effects could be reduced.
Experimental procedure
The effect of holding an AI alloy melt under a vacuum
In order to determine the effect of holding an Al alloy melt under a vacuum on the oxide film content, 6 kg of Al-7Si-0.3Mg (wt%) (2L99) alloy was melted in an induction furnace and then allowed to solidify under a reduced pressure of 80 mbar. The solidified casting was sectioned into two halves, and the internal surfaces of the pores were investigated using scanning electron microscopy (SEM) (Philips XL-30 instrument) with energy dispersive spectroscopy (Oxford Inca) to determine their relationship to double oxide film defects.
Investigation of the effect of holding time before solidification on double oxide film defects
Castings were produced by the investment casting technique, and contained oxide films which were nominally of different ages; 0, 10, and 20 minutes, by holdup at these times. Three different aluminium alloys were considered: commercial-purity Al, Al-7Si-0.3Mg (wt%), and Al-5Mg (wt%) to involve different oxide films that might have different behaviours. The three oxide species expected to form in each alloy were Al2O3, MgAl2O4, and MgO.
In each experiment, about 10 kg of the given alloy was melted and held at about 800°C under a vacuum of about 80 mbar for one hour. This was intended to remove previously introduced oxide films from the melt (Raiszadeh and Griffiths, 2010; El-Sayed, Hassanin, and Essa, 2016). The liquid metal was then poured into preheated investment shell moulds, which were placed in an induction furnace and stirred using a power setting of 7.5 kW and frequency of 2350 Hz for one minute. This led to splashing of the liquid metal surface, and the creation of new double oxide film defects and their entrainment into the melt.
One casting was then allowed to solidify immediately, while two further castings were maintained in the liquid state by placing the filled ceramic shell mould in a furnace for 10 and 20 minutes, respectively, then removing it and allowing the melt to solidify. The change in hydrogen content of each melt during holding was also determined. After solidification, each of the castings was machined into 15 tensile test bars for determination of the ultimate tensile strength and percentage elongation at failure, using a cross-head speed of 0.17 mm.s-1. The tensile results were evaluated using a Weibull statistical analysis approach (Weibull, 1951) to assess the influence of the holding period on the variability of the mechanical properties of the castings. Finally, the fracture surfaces of the test bars were examined using SEM.
The effect ofholding time on the composition ofan air bubble
In order to follow the changes in gas composition of the internal atmosphere of a double oxide film defect within an Al melt, a series of analogue experiments was carried out to determine the changes in composition of a trapped air bubble held in a melt of commercial-purity Al. The air bubble was formed by immersing a blind hole of 6 mm diameter and 5 mm depth, drilled into the centre of a steel piece, into an Al melt. This was then rotated at a speed of 540 r/min, corresponding to an angular velocity of 1.4 m.s-1. The air bubble inside the hole was therefore in direct contact with the Al melt, allowing its interior (initially air) to react with the surrounding liquid Al and hydrogen to diffuse between the melt and air bubble. Two such experiments were carried out, of 5 and 20 minutes duration, before allowing the melt to solidify. After solidification, a section containing the remaining air bubble was machined out of the casting, without piercing the bubble. This section was placed in a pore gas analyser (constructed by Hyden Ltd), in which the contents of the bubble were extracted under a vacuum, and the gas obtained analysed using mass spectrometry. A reference air bubble containing ambient atmosphere was created by soldering and sealing the bottom of a blind hole of the same dimensions, which was also analysed to provide a calibration sample that should comprise the normal composition of the atmosphere.
Results
The effect of holding liquid Al under a vacuum
Figure 3 shows an example of an SEM image from within a pore that was allowed to solidify under a vacuum of about 80 mbar. Oxide fragments were visible inside the pore and were confirmed by energy-dispersive X-ray (EDX) analysis, which indicated the presence of MgAl2O4 spinel. This suggests that the origin of the pore lay with a double oxide film defect. Holding the melt under vacuum would be expected to cause the expansion of any atmosphere within the entrained double oxide films, increasing their buoyancy and causing them to float to the surface of the melt, thus reducing their harmful effects on the mechanical properties of the casting. In this experiment, the holding process was interrupted and the liquid metal was allowed to solidify under vacuum in order to determine the character of the porosity inside the casting. The analysis of these pores confirmed a relationship with oxide films. Subsequently, the melts used to make the castings for investigation of the effect of holding time on their scatter of properties were subjected to a vacuum for 1 hour before casting, in order to minimize the effect of prior oxide film defects.
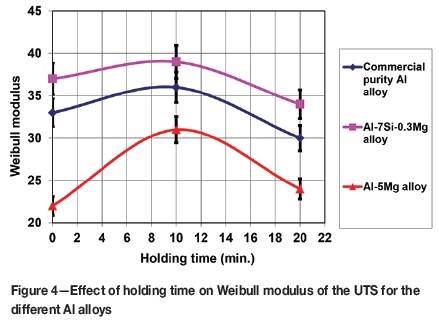
The effect of holding time before solidification on mechanical properties
Table I shows the Weibull analysis of the mechanical property results of the three different alloys, as well as measurements of the melt hydrogen content. The Weibull moduli of the UTS are also shown in Figure 4. In all three alloys, the Weibull moduli were maximum for the castings held for 10 minutes before solidification, although for the pure aluminium and Al-7Si-0.3Mg alloys the maximum values were only slightly higher than the Weibull moduli at 0 and 20 minutes. The hydrogen content of the castings increased with holding time, with this being particularly marked for Al-5Mg, due to the greater solubility of hydrogen in this alloy (Anyalebechi, 1995).
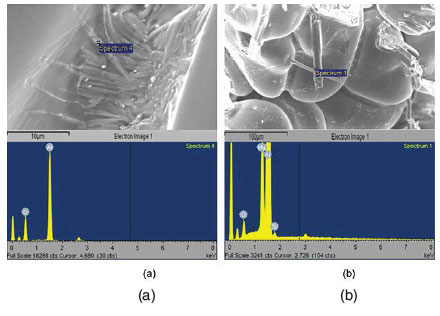
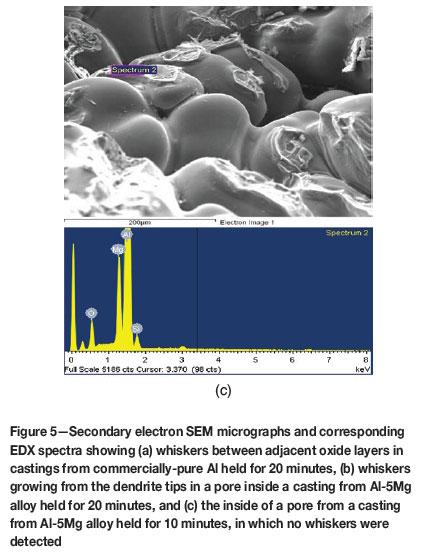
Figures 5a and 5b show whisker-like oxides found within some pores of the commercial-purity Al and the Al-5Mg alloy castings held for 20 minutes before solidification. The pores are suggested to be associated with oxide films, as shown by EDX analysis which indicated the presence of Al2O3 and/or MgO inside the pores. Although the interconnections were too small to influence the mechanical properties, their presence perhaps suggests that chemical reactions were occurring within the pore atmosphere, resulting in the deposition of ceramic whiskers. No whiskers were found on the fracture surfaces of samples, either when solidified immediately or when held in the liquid state for 10 minutes before solidification. This is shown in Figure 5c, which illustrates a casting from Al-5Mg alloy held for 10 minutes before solidification.
Results of the pore gas analysis experiments
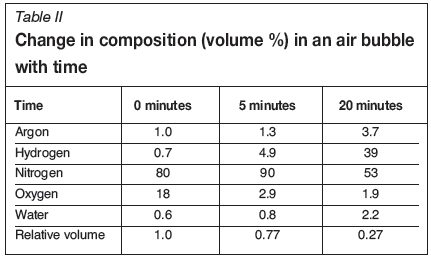
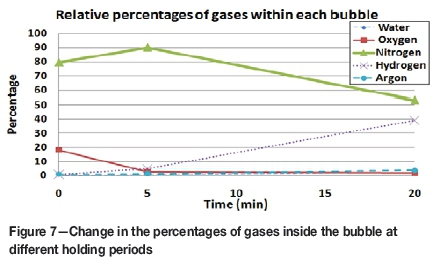
Figure 6 depicts the initial results from the pore gas analysis experiment, showing the composition of gas bubbles held for different times in liquid metal subjected to stirring at 540 r/min. The change in composition with time is given in Table II, and shown in Figure 7. The data suggests that the first 5 minutes of the experiment were characterized by a rapid loss of oxygen (causing the air bubbles to lose most of their oxygen content within this holding period) and a slight increase in hydrogen content. The slight increase in nitrogen content was presumably due mostly to the reduction in bubble volume as the oxygen reacted with the liquid Al. After 20 minutes' holding, the bubble volume was reduced to about one-quarter of its original size, as shown by the increase in argon content, which is unreactive and insoluble in liquid Al, but by this time almost all of the initial oxygen content had been consumed. The nitrogen content was also reduced to about 20% of its initial amount, but the hydrogen concentration had increased markedly, to represent about 40% of the volume of the bubble. The the bubble in the melt held for 20 minutes therefore consisted mainly of nitrogen and hydrogen with trace amounts of oxygen, argon, and water vapour.
Discussion
Previous research suggested that holding liquid metal under vacuum may cause its entrained bifilms to expand (Dispinar and Campbell, 2004; Fox and Campbell, 2000). The SEM investigation accompanied by EDX analysis (Figure 3) detected many oxide fragments inside the pores in the casting solidified under vacuum. The fragments were determined to be spinel, suggesting that such pores were initially double oxide film defects that expanded due to the application of the vacuum. If the liquid aluminium is held under vacuum for a sufficient time, this might allow double oxide film defects to expand and float to the melt surface. In the experiment performed here, the holding treatment was suspended at an intermediate stage to allow investigation of the defects. The significance of this experiment is that, since the experiments involving the holding of castings in the liquid state were made with melts previously held under a vacuum, the oxide film defects seen in the mechanical property test bars were probably created during casting of the bars, rather than the result of prior casting processes.
The mechanical property data shown in Table I indicates a peak in properties in the casting held for 10 minutes before solidification. This could be an indication of two competing mechanisms, each influencing the morphology of the double oxide film defects, (i.e., their size and shape), and hence their effect on mechanical properties. Initially, the internal atmosphere of the double oxide film defects may have been reduced by reaction of their oxygen content with the surrounding melt, and as the volume of the internal atmosphere decreased, the size of the defects, and their effect on mechanical properties, was correspondingly reduced, resulting in an increase in the Weibull modulus to reach a maximum at 10 minutes. The second effect may be the diffusion of hydrogen from the melt into the defect interiors, increasing their size and the effect on mechanical properties, and so decreasing the Weibull modulus.
The whisker-like structures found within pores, shown in Figure 5 and confirmed by EDX to be oxides, occurred mostly at holding periods of 20 minutes. The whisker-like growths are indicative of ceramic structures grown from a vapour phase (Edwards and Happel, 1962; Hayashi and Saito, 1974), which might be evidence that they formed within porosity containing an atmosphere. The occurrence of whiskers in the pores in the castings, (which were themselves related to oxides) is suggestive of double oxide film defects retaining an atmosphere for tens of minutes after their formation, and perhaps an atmosphere consisting mostly of hydrogen. DeVries and Sears (1959) reported that heating of alumina to 2000°C in the presence of hydrogen caused the vaporization of alumina to produce Al2O and water vapour, both in the gaseous form. When the temperature was lowered, the reaction was reversed and alumina was deposited in the form of whiskers. It is speculated that, during holding of the melt, reaction between the bifilm atmosphere and the surrounding melt may produce sufficient heat energy to locally increase the temperature of the double oxide film defect to such an extent that could cause the partial reduction of the alumina forming the bifilm to Al2O (which would be expected to occur as a vapour phase at these temperatures) and water vapour. During solidification, if all of the O2 and N2 had been consumed, the temperature would begin to decrease, perhaps resulting in the reaction of Al2O with water vapour to produce the alumina in whisker form.
The effect of holding time on the composition of the internal atmosphere of a double oxide film defect is supported by the initial results of the pore gas analyses shown in Table II and Figure 7. The most marked changes in the bubble composition were the loss of oxygen during the early stages of holding, and the subsequent increase in hydrogen content.
To summarize, the change in mechanical properties, the occurrence of oxide whiskers, and the pore gas analysis results suggest that double oxide films, once formed, quickly undergo changes in their internal atmosphere which affect the mechanical properties by influencing the size and shape of the double oxide films. These changes comprise the rapid consumption of oxygen and a slower accumulation of hydrogen, with the latter dependent on the hydrogen content of the melt. The consumption of nitrogen was also a slower process, occurring subsequent to the reaction of oxygen, although complete oxygen consumption did not appear to be required before the formation of AIN.
Conclusions
► Holding Al castings in the liquid state for up to 20 minutes before solidification resulted in peak values of the Weibull modulus occurring at a nominal holding period of 10 minutes
► Whisker-like structures of oxides were found in pores in castings solidified after holding for 20 minutes in the liquid state
► Pore gas analysis showed that a trapped air bubble held in stirred liquid Al alloy lost most of its oxygen content within around 5 minutes, and subsequently gained hydrogen by diffusion from the melt
► These results suggest that double oxide film defects in liquid Al alloys may have variable effects on casting properties, depending on their morphology. This morphology is influenced by the composition of the interior atmospheres of the defects, which is influenced by reaction with the surrounding melt and diffusion of hydrogen into the defect.
References
Anyalebechi, P.N. 1995. Analysis of the effects of alloying elements on hydrogen solubility in liquid aluminum alloys. Scripta Metallurgien et Materialia, vol. 33. pp. 1209-1216. [ Links ]
Basuny, F.H., Ghazy, M., Kandeil, A.-R.Y., and El-Sayed, M.A. 2016. Effect of casting conditions on the fracture strength of Al-5 Mg alloy castings. Advances in Materials Science and Engineering. DOI: 10.1155/2016/6496348 [ Links ]
Campbell, J. 2003. Castings. Butterworth-Heinemann. [ Links ]
Campbell, J. 2006. Entrainment defects. Materials Science and Technology, vol. 22. pp. 127-145. [ Links ]
Cao, X. and Campbell, J. 2003. The nucleation of Fe-rich phases on oxide films in Al-11.5Si-0.4Mg cast alloys. Metallurgical and Materials Transactions, vol. 34A. pp. 1409-1420. [ Links ],
Deveries, R.C. and Sears, G.W. 1959. Growth of aluminum oxide whiskers by vapor deposition. Journal of Chemical Physics, vol. 31. pp. 1256-1257. [ Links ]
Dispinar, D. and Campbell, J. 2004. Critical assessment of reduced pressure test. part 1: porosity phenomena. International Journal of Cast Metals Research, vol. 17. pp. 280-286. [ Links ]
Edwards, P.L. and Happel, J.R.J. 1962. Alumina whisker growth on a single- crystal alumina substrate. Journal of Applied Physics, vol. 33. pp. 826-827. [ Links ]
El-Sayed, M.A. 2015. Effect of welding conditions on the mechanical properties of friction stir welded 1050 aluminum alloy. International Review of Mechanical Engineering, vol. 9. pp. 252-256. [ Links ]
El-Sayed, M.A. 2016. The behaviour of bfilm defects in cast Al-7Si-Mg alloy. PloS one, vol. 11, no. 8. e0160633. DOI: 10.1371/journal.pone.0160633 [ Links ]
El-Sayed, M.A. and Griffiths, W.D. 2014. Hydrogen, bfilms and mechanical properties of Al castings. International Journal of Cast Metals Research, vol. 27. pp. 282-287. [ Links ]
El-Sayed, M.A., Hassanin, H., and Essa, K. 2016. Effect of casting practice on the reliability of Al cast alloys. International Journal of Cast Metals Research, pp. 1-5. DOI: 10.1080/13640461.2016.1145966 [ Links ]
Fox, S. and Campbell, J. 2000. Visualisation of oxide film defects during solidification of aluminium alloys. Scripta Materialia, vol. 43. pp. 881-886. [ Links ]
Hayashi, S. and Saito, H. 1974. Growth of magnesia whiskers by vapor phase reactions. Journal of Crystal Growth, vol. 24-25. pp. 345-349. [ Links ]
Nyahumwa, C., Green, N.R., and Campbell, J. 1998a. The concept of the fatigue potential of cast alloys. Journal of the Mechanical Behavior of Materials, vol. 9. pp. 227-235. [ Links ]
Nyahumwa, C., Green, N.R., and Campbell, J. 1998b. Effect of mold-filling turbulence on fatigue properties of cast aluminum alloys. AFS Transactions, vol. 106. pp. 215-223. [ Links ]
Raiszadeh, R. and Griffiths, W.D. 2006. A method to study the history of a double oxide film defect in liquid aluminum alloys. Metallurgical and Materials Transactions B, vol. 37. pp. 865-871. [ Links ]
Raiszadeh, R. and Griffiths, W.D. 2008. A semi-empirical mathematical model to estimate the duration of the atmosphere within a double oxide film defect in pure aluminum alloy. Metallurgical and Materials Transactions B, vol. 39. pp. 298-303. [ Links ]
Raiszadeh, R. and Griffiths, W.D. 2010. The behaviour of double oxide film defects in liquid Al alloys under atmospheric and reduced pressures. Journal of Alloys and Compounds, vol. 491. pp. 575-580. [ Links ]
Weibull, W. 1951. A statistical distribution function of wide applicability. Journal of Applied Mechanics, vol. 13. pp. 293-297. [ Links ]
Youssef, Y. and El-Sayed, M. 2016. Effect of reinforcement particle size and weight fraction on the mechanical properties of SiC particle reinforced Al metal matrix composites. International Review of Mechanical Engineering, vol. 10, no. 4. pp. 261-265. DOI: 10.15866/ireme.v10i4.9509 [ Links ]
Paper received Apr. 2016
Revised paper received Nov. 2016