Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 no.6 Johannesburg Jun. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n6a10
PAPERS OF GENERAL INTEREST
Synthesis of 3-hydroxy-2-naphthyl hydroxamic acid collector: flotation performance and adsorption mechanism on bastnaesite
Z. Yang; W. Wu; X. Bian
School of Metallurgy, Northeastern University, Shenyang 110819, Liaoning,China
SYNOPSIS
The synthesis of 3-hydroxy-2-naphthyl hydroxamic acid (H2O5) as a collector for bastnaesite is described. The flotation performance of H2O5 with respect to bastnaesite was investigated by flotation tests, measurements of the surface adsorption capacity, and polarizing microscopy. The results indicated that H2O5 exhibits superior collecting performance compared with direct flotation recovery of bastnaesite, with recoveries above 90% at pH 8-9. The adsorption mechanism of H2O5 on bastnaesite was studied by solution chemistry analysis and Ç-potential tests. The results showed that the adsorption of H2O5 is associated with the types and concentrations of hydrolysis products of rare earth cations on the bastnaesite surface. At pH 8-9, the hydrolysis-dominant components RE(OH)2+ and RE(OH)2+ of the rare earth cations were adsorbed onto the bastnaesite surfaces, which were considered to be the major positive active points and were beneficial to chemical adsorption of the reagents. The pH range of 7-10 was optimum for bastnaesite floatability, and the adsorption of H205 on bastnaesite was by means of chemical adsorption through formation of a five-membered ring chelate.
Keywords: 3-hydroxyl-2-naphthyl hydroxamic acid (H205), flotation, bastnaesite, chemical adsorption.
Introduction
The rare earth elements (REEs) have long been known as 'industrial gold' and are widely used in the petroleum, chemical, ceramic, metallurgical, and permanent magnet material industries because of their special physicochemical properties (Chen, 2011; Krishnamurthy and Guptac, 2002; Esquivel, Bohe, and Pasquevich, 2002; Abrahami, Xiao, and Yang, 2015). Demand for REEs is growing in response to continuous advances in science and technology. Currently, most REEs are extracted from bastnaesite ((Ce,La)CO3F) (Chelgani et al., 2015; Yosry, 1990), which is recovered from rare earth ores by flotation. Typically, hydroxamic acids are used as bastnaesite collectors (Xu, 2015; Ren, 1997; Mousumi and Venugopal, 2016). Research into the mechanism of interaction between hydroxamic acids and bastnaesite is important for improving the recoveries of bastnaesite. However, there have been few studies that examine this mechanism.
Compared to other available collectors, hydroxamic acid has been shown to have better selectivity for bastnaesite. Hydroxamic acids (R-CO-NHOH) are much weaker acids than the corresponding carboxylic acids with identical carbon chains (Jiang et al., 2010; Buglyo et al., 2007; Wu and Zhu, 2006; Herrera, 2003). However, hydroxamic acids are better at selecting chelating metal ions (Wang, Liu, and Miller, 2008; Griffith et al., 2011). The two O atoms in the carbonyl and hydroxyl groups of a hydroxamate molecule bond with a metal cation to form a five-membered ring structure (Chelgani et al., 2015). Researchers have reported on the surface complexes of hydroxamic acid and metal species on the mineral surfaces (Jiang et al., 2010; Wu and Zhu, 2006). This research has led to the wide use of hydroxamic acids such as octyl hydroxamic acid and salicylhydroxamic acid as collectors for flotation recovery of metal oxide minerals, including copper (Lee, 1998), tin (Sreenivas and Padmanabhan, 2002) and rare earth minerals (Zhou et al., 2015a; Jordens et al., 2014).
Flotation is a complex physical and chemical process in which collectors play a decisive role. Flotation is a useful approach for fine-grained rare earth occurrences. Other physical separation methods (e.g. magnetic, gravity) have been proven to be impractical. There have been a number of investigations of flotation processes for bastnasite ores (Cai et al., 2009). In each case conditions, including a suitable collector and pH, have been established for the flotation of the desired minerals and depression of unwanted minerals.
In our study, we used H205, which was synthesized in a laboratory, as a hydroxamic acid rare earth collector. We investigated the effects of H205 dosage and the flotation pH on pure bastnasite. Further, we studied the collecting property and adsorption mechanism of H205 on the surface of bastnasite.
Materials and methods
Materials
Minerals
Hand-picked, high-purity bastnaesite was obtained from Baotou, Mongolia, China, and ground using a porcelain mill. The ground samples were wet-sieved to separate the particles that were less than 74µmfor flotation tests. A portion of this fraction was further ground in an agate mortar to obtain particles less than 5 µm for the zeta potential measurements.
Reagents
Analytical-grade HCl and NaOH were used as pH modifiers. Analytical-grade 3-hydroxy-2-naphthyl methyl acid, NH2OH.HCl, methanol, and H2SO4 were used to synthesize the collector. Distilled water with a pH of 6 to 7 was used to prepare all reagent solutions and for the flotation tests. Pine oil provided by Baogang Corporation (Inner Mongolia, China)was used as a frother. The 3-hydroxy-2-naphthyl hydroxamic acid collector was synthesized in the laboratory using the following method.
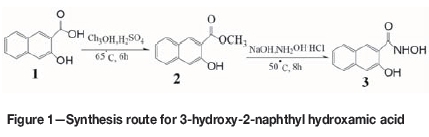
► Synthesis of 3-hydroxy-2-naphthyl methyl acid methyl ester-3-hydroxy-2-naphthyl methyl acid (1; 18.8 g, 0.1 mol) was reacted with methanol (16.0 g, 0.5 mol) in the presence of concentrated sulphuric acid (0.76 mL, 0.014 mol) at 65°C for 6 hours. The unreacted methanol was then removed under reduced pressure and 3-hydroxy-2-naphthyl acid methyl ester (2; 17.3 g) was obtained with 85.2% yield.
► Synthesis of 3-hydroxy-2-naphthyl hydroxamic acid- Sodium hydroxide (8.8 g, 0.2 mol) and hydroxylamine hydrochloride (7.64 g, 0.1 mol) were dissolved in water (50 mL). Within 0.5 hours, 3-hydroxy-2-naphthyl acid methyl ester (2; 20.3 g, 0.1 mol, dissolved in 60 mL methanol) was added at 50°C. The mixture was stirred for 8 hours at 50°C, then methanol was removed from the reaction mixture under reduced pressure, the residue was acidized with 3 mol/L HCl, and 3-hydroxy-2-naphthyl hydroxamic acid (3;16.4 g) was obtained with 71.2% yield.
The synthesis route is shown schematically in Figure 1.
Experimental
Flotation tests
Flotation tests were carried out using an XFD-0.1L flotation machine (mechanical agitation) with a 100 mL plexiglass cell. The impeller speed was fixed at 1500 r/min. The pH values of the pulp were measured by a PHS-3E pH meter. All the flotation tests were carried out at 25°C. For each test, 20.0 g mineral samples were placed in the cell with 100 mL distilled water at 25°C. After adding the required amount of collector (H205), the pulp was agitated for 2 minutes and the pH was then adjusted to a designated value with hydrochloric acid or sodium hydroxide solution before flotation. Flotation was conducted for 5 minutes. The concentrates and tailings were weighed after filtration and drying, respectively.
Zeta potential tests
The zeta potential tests were carried out using a JS94H electrophoresis apparatus (Beckman Coulter Inc., USA). 100 mg of -5 )im mineral samples were added to 40 mL of aqueous solution with or without 0.6 g/L H205, and then stirred for 15 minutes. The pH values were adjusted using HCl or NaOH solutions. Each sample was measured five times independently, and the average value was taken. For pH measurements, the samples were allowed to equilibrate for 5 minutes before measurement. To avoid zeta potential hysteresis, fresh samples were prepared for acidic and basic zeta potential measurements at 25°C.
Adsorption ofH205 on the mineral surface
The H205 concentration in the aqueous solutions was measured by a UV-V double-beam UV-2100 spectrophotometer from Labtech Co. (China). Bastnaesite samples (1.0 g) with 0.6 g/L of H205 were measured at different pH values. The residual concentration of the surface collector solution was measured and then converted to the amount of H205 adsorbed on the bastnaesite surfaces. The samples were stirred for 5 minutes at 25°C, at a certain pH value, and then filtered and the residual concentration of H205 in the filtrate measured.
Results and discussion
Flotation results
Figure 2 showed the relationship between the recovery of bastnaesite and H2O5 dosage when the pH was adjusted to 8 using sodium hydroxide. The recovery increased with increasing H2O5 concentration, reaching a maximum of about 96% at an H2O5 dosage of 0.6 g/L. This demonstrates that bastnaesite has good floatability in the H2O5 flotation system.
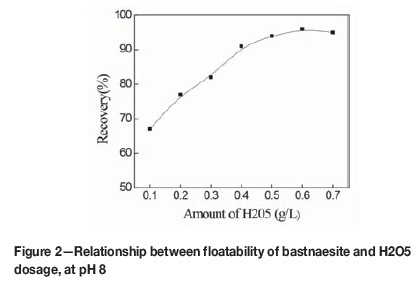
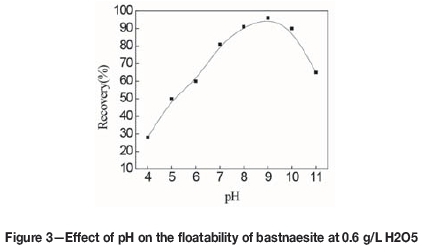
Figure 3 shows the effect of pH on bastnaesite recovery at an H2O5 dosage of 0.6 g/L. At pH values of 7-10, the bastnaesite retained relatively good floatability and the recovery rates were all above 80%.
Polarizing microscopy
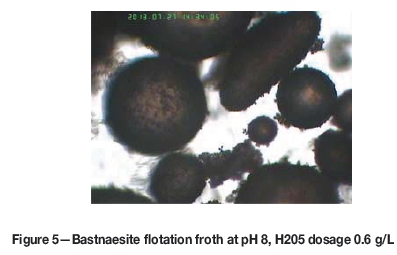
Figures 4-6 show images of bastnaesite flotation froth at an H205 dosage of 0.6 g/L and at pH values of 2, 8, and 13, respectively. It can be seen that many bastnaesite particles adhered to the bubble surfaces at pH 8, whereas the particles did not adhere to the bubble surfaces at pH 2 and pH 13.


Amount ofH205 adsorption on bastnaesite surfaces
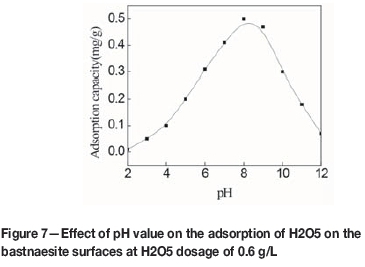
Figure 7 shows the effect of pH on the adsorption of H2O5 on the bastnaesite surfaces at an initial collector concentration of 0.6 g/L. At pH 2, adsorption of H2O5 was approximately zero. With increasing pH the adsorption rose sharply, reaching a maximum at pH 8, and then decreasing sharply to approximately zero at pH 12. Thus, it can be seen that the preferable pH range for H2O5 adsorption on the bastnaesite surfaces is 7 to 9, which is in accordance with the results of the flotation tests.
Solution chemistry analysis of the flotation process
H2O5 is a very weak acid - even weaker than carbonic acid (Xu, Xu, and Wang, 2002; Chi and Wang, 2014). According to the soft and hard acid-alkaline (HSAB) theory (Xu, Xu, and Wang, 2006), hydroxamic acid forms relatively unstable chelates with alkali metals, alkaline earth metals, and other ions. However, it forms relatively stable chelates with transition metals such as the REEs, iron, copper, and other hard acid metal ions. In these chelates, the chemical bonds between the metal ions and the hydroxamic acid are substantially coordinate-covalent bonds (Che et al., 2004a, 2004b). The crystal surface characteristics of the minerals are closely associated with the floatability. The hydroxamic acid and lattice ions on the surfaces of the rare earth minerals are formed into a complex with a coordinate-covalent bond. This complex is strongly hydrophobic, and renders the minerals floatable when the mineral surfaces are covered. The chelate formed by the complex and the alkali metals or alkaline-earth metals and other ions is of an ionic type and is weakly hydrophobic, and therefore has relatively poor floatability in hydroxamic acid systems (Fuerstenau, 1985; Jordens, Chen, and Waters, 2013; O'Brien et al., 1997; Sreenivas and Padmanabhan, 2002). According to this principle, using H2O5 as the collector, a rare earth concentrate suitable for further processing (above 50% rare earth oxides) can be obtained from complex rare earth ores by flotation.
In the flotation pulp system the conditions for interaction between the flotation reagents and the minerals are determined by the dissolution of the minerals, dissociation reactions of the reagents, hydrolysis reactions of the dissolved components, and chemical reactions of the flotation reagents. The chemical behaviour of the solution dominates the conditions and various balanced relations of these reactions (Ananthapadmanabhan and Somasundaran, 1985).
Once the minerals contact water, they will be dissolved. This is relevant to the properties of both the minerals and the solution. The dissolved mineral components will also undergo different chemical reactions to form a variety of chemical components, thereby affecting the surface properties of the minerals (Pugh, 1991).
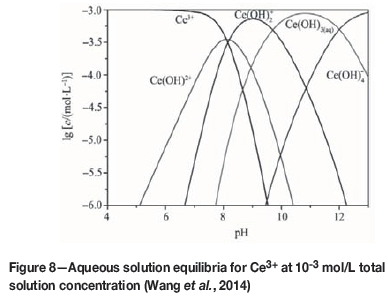
After bastnaesite has been cracked and dissociated, rare earth cations are exposed on the surfaces of the lattices. Studies have shown that these ions have a relatively high hydration energy and easily undergo hydration in aqueous solutions to form hydroxyl-complex ions with strong surface activities, and can be strongly adsorbed in the compact layer (Stern layer) of the double electrode layer of bastnaesite, which affects the surface zeta potential of bastnaesite (Fuerstenau, 1983). In aqueous solutions, the rare-earth metal cations are hydrolysed. Ce3+ can be taken as an example. Figure 8 shows the hydrolytic equilibrium diagram in the aqueous solution at a Ce3+ concentration of 1x10-3 mol/L.
The hydrolysis reaction of Ce3+ in the aqueous solution can be written as follows (Ren et al., 1997):
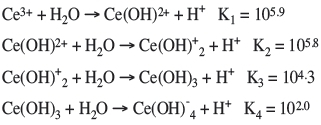
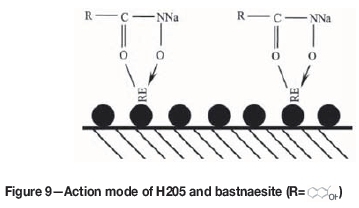
As shown in Figure 8, different hydrolysis products of the rare earth cations predominate in aqueous solution at different pH conditions, and their levels of concentration affect the chemical adsorption of the reagents. At pH<9, the dominant hydrolysis components are Ce(OH)2+, Ce(OH)2+, and Ce 3+. At pH>9, the dominant hydrolysis components are Ce(OH)3 and Ce(OH)4-, and the concentrations of Ce(OH)2+ and Ce 3+ began to decrease. At pH 8-9, the dominant components are Ce(OH)2+ and Ce(OH)2+. This pH range is also the best for bastnaesite flotation (as shown in Figure 3). Hydroxamic acid anions dissociated from H2O5 undergo a chemical adsorption reaction with the hydroxy complexes on the surfaces of bastnaesite to form a stable five-membered ring complex, as shown in Figure 9. As a result, bastnaesite became hydrophobic (He and Vaisey, 2012). The OH-concentration in the pulp gradually increased at pH>9. According to the 'chemical reaction hypothesis' or the 'solubility product hypothesis' proposed by Taggart and Plaksins for flotation reagents (Hu, 2014), a Ce(OH)3 cover film forms on the surfaces of bastnaesite, which prevents the combination of hydroximic acid ions with rare earth ions and reduces the recovery rate of bastnaesite. In an acidic medium (pH<7), the dominant hydrolysis component of the rare earth ion is Ce3+. Theoretically, hydroximic acid could react with Ce3+ to form the chelate (Liu et al., 1989). However, in this pH range bastnaesite had very poor floatability (as shown in Figure 3), indicating that the dominant hydrolysis component, Ce3+, was unfavourable to flotation. According to the coordinated complexation reaction theory of coordination chemistry (Luo, 2012), as the pH of the flotation pulp is adjusted by addition of hydrochloric acid, Cl- and hydroxamic acid in the pulp are adsorbed onto the surfaces of the minerals competitively to reduce the floatability. Furthermore, because hydroxamic acid is weaker than carbonic acid, when the pH is lowered, fewer hydroxamic acid ions dissociate from the pulp. According to the coordinated complexation reaction theory (Luo, 2012), when the concentration of ionic hydroxamic acid was reduced, the coordination capability with the ions on the bastnaesite surfaces was also lowered. This means that any acidic conditions are unfavourable for bastnaesite floatation.
Zeta potential analysis
Figure 10 shows the relationship between the zeta potential and pH. The isoelectric point (IEP) of pure bastnaesite was at pH 8.1. After bastnaesite reacted with H2O5, the zeta potential moved in the negative direction and the IEP moved from pH 8.1 to 6.6, which suggests that H205 ions were adsorbed on the bastnaesite surfaces. The collector was adsorbed on the mineral surfaces in an ionic form by either chemical adsorption or physical adsorption as a result of electrostatic attraction forces. In the pH range greater than the IEP, H2O5 could still be adsorbed onto the surfaces of the negatively charged bastnaesite to produce a higher negative potential. Therefore, we can conclude that H2O5 ions were not electrostatically adsorbed onto the surfaces of the bastnaesite, but entered the compact double-electrode layer of the bastnaesite by chemical adsorption.
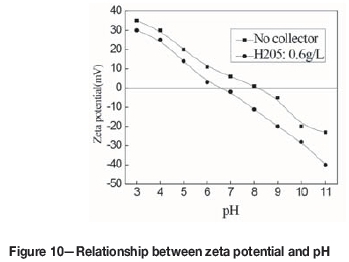
Zeta potential that becomes more negative in the presence of a collector is associated with the compositions of both collector and the bastnaesite-water interface at different pH values. The organic compounds that contain hydroxamic acid groups (-CONHOH) have an affinity for REEs (Zhou, 2015). Since the pKa of H205 is about 7.0 (Che et al., 2004a, 2004b), the polar group in H205 registers mainly as neutral hydroxamic acid groups (-CONHOH) at pH <7.0, and the H205 anion (C(O-)N(O-)) at pH >7.0. When H205 was introduced into the pulp at different pH values, its species with more negative charge possibly replaced H2O, OH-, or O2-in the bastnaesite/water interface, thus resulting in the zeta potential of the minerals declined (Zhou et al., 2015a, 2015b; Jiang, Li, and Feng, 2011). The IEP of bastnaesite was at pH 8.0 (as shown in Figure 10). Since both bastnaesite and H205 are negatively charged at pH >8.0, the adsorption of H205 on the surfaces of bastnaesite has to overcome the electrostatic repulsion. However, H205 could be adsorbed on the bastnaesite surfaces at pH >8.0, so the collection of H205 to bastnaesite was a result of the chemical adsorption, which formed a five-membered ring chelate. At pH<8.0, because the IEP of bastnaesite in the absence of H205 was about pH 8.0. (as shown in Figure 10), bastnaesite is positively charged. H205 may adsorb on the bastnaesite surfaces by physical adsorption or chemical adsorption. The change in zeta potential could reflect the interaction between collector and mineral. In the presence of H205, the zeta potential of bastnaesite particles became more negative, inferring that H205 can adsorb onto bastnaesite surfaces.
Conclusions
1. H2O5 has a good collecting property for bastnaesite. The bastnaesite has relatively good floatability in the range of pH 7-10 and the best floatability at approximately pH 9
2. Chemical analysis of the mineral solution showed that the types and concentrations of the hydrolysis products of the rare earth cations on the surfaces of bastnaesite affect the floatability of the minerals. In aqueous solution at pH 8-9, the major hydrolysis products RE(OH)2+ and RE(OH)2+ are the main positive active points and are adsorbed to the surfaces of the bastnaesite. At pH <7, the major hydrolysis product is RE3+, which is unfavourable for collection and considered to be the main negative active point. At pH >9, the major hydrolysis products are RE(OH)3 and RE(OH)4-, which are adsorbed onto the bastnaesite surfaces. This is unfavourable for floatation and is also considered to be a main negative active point
3. Zeta potential analysis indicates that at pH 8-9, H2O5 is adsorbed chemically on the surface of the bastnaesite to form stable five-membered ring chelate. At pH <8.0, bastnaesite is positively charged and H205 may adsorb on the mineral surfaces by either physical or chemical adsorption.
4. Surface adsorption measurements indicate that the preferable pH range for H2O5 adsorption on bastnaesite surfaces is pH 7-10. H2O5 adsorption reaches its maximum value at pH 8.
Bastnaesite is a typical oxidized mineral, which is usually associated with fluorite, apatite, haematite, and quartz. According to our conclusions, H2O5 has a good collecting property for bastnaesite. The flotation performance of bastnaesite with H2O5 was best at pH 8-9. Water glass and sodium carboxymethyl cellulose (CMC) as depressants of gangue minerals (fluorite, apatite, haematite, and quartz), with H2O5 as collector, are recommended for bastnaesite flotation.
Open- and closed-circuit flotation experiments were carried out under the optimum conditions so as to simulate the industrial flotation process. The factors influencing the flotation process, such as the pulp concentration, mineral particle size, flotation temperature, and H205, depressant, and frother additions, were investigated to establish the optimum flotation conditions.. Preliminary tests covering gravity and magnetic separation were also conducted. Although flotation is the main concentration method for rare earth ores, other unit operations such as magnetic and gravity separation prior to flotation may obtain better results.
Acknowledgments
The authors acknowledge the support of the Major State Basic Research Development Program of China (973 program) (2012CBA01205).
References
Abrahami, S.T., Xiao, Y., and Yang, Y. 2015. Rare-earth elements recovery from post-consumer hard-disc drives. Mineral Processing and Extractive Metallurgy, vol. 124, no. 2. pp. 106-115. [ Links ]
Ananthapadmanabhan, K.P. and Somasundaran, P. 1985. Surface precipitation of inorganics and surfactants and its role in adsorption and flotation. Colloids and Surfaces, vol. 13. pp. 151-167. [ Links ]
Buglyo, P., Nagy, E.M., Farkas, E., Sovago, I., Sanna, D., and Micera, G. 2007. New insights into the metal ion-peptide hydroxamate interactions: metal complexes of primary hydroxamic acid derivatives of common dipeptides in aqueous solution. Polyhedron, vol. 26. pp. 1625-1633. [ Links ]
Cai, Z.L., Cao, M.L., Che, L.P., Yu, Y.F., and Hu, H.Y. 2009. Study on the beneficiation process for recovering rare-earth from the LIMS tailing of HIMS rougher concentrate after magnetizing roasting in Baogang concentrator. Metal Mine, vol. 7. p. 155 (in Chinese). [ Links ]
Chelgani, S., Chehreh A., Rudolph, M.A., Leistner, T.A., and Gutzmer, J.A. 2015. A review of rare earth minerals flotation: monazite and xenotime. International Journal of Mining Science and Technology, vol. 25. pp. 877-883. [ Links ]
Che, L.P., Yu, Y.F., Pang, J.X., Yuan, J.Z., and Zhang, F.G. 2004a. Application and trend of hydroximic acid as collectors on flotation of RE minerals. Chinese Rare Earths, vol. 25, no. 3. pp. 49-54 (in Chinese). [ Links ]
Che, L.P., Yu, Y.F., Pang, J.X., Yuan J.Z., and Wang X.T. 2004b. Synthesis and properties of hydroxamic acid collectors and their mechanism in the flotation of rare earth minerals. Chinese Rare Earths, vol. 25, no. 6. pp. 74-81 (in Chinese). [ Links ]
Chen, Z.H. 2011. Global rare earth resources and scenarios of future rare earth industry. Journal of Rare Earths, vol. 29, no. 1. pp. 1-5. [ Links ]
Chi, R.A. and Wang, D.Z. 2014. Mineral Processing of Rare Earth. 1st edn. Science Press, Beijing, China (in Chinese). [ Links ]
Esquivel, M.R., Bohe, A.E., and Pasquevich, D.M. 2002. Carbochlorination of cerium dioxide. Mineral Processing and Extractive Metallurgy Review, vol. 111, no. 3. pp. 149-155. [ Links ]
Fuerstenau, D.W. 1985. Adsorption of hydroxamate collectors on semisoluble minerals. Part II: Effect of temperature on adsorption. Colloids and Surfaces, vol. 15. pp. 137-146. [ Links ]
Fuerstenau, D.W. 1983. The adsorption of hydroxamate on semi-soluble minerals. Part I: Adsorption on barite, calcite and bastnaesite, Colloids and Surfaces, vol. 8. pp. 103-119. [ Links ]
Griffith, D.M., Szocs, B., Keogh, T., Suponitsky, K.Y., Farkas, E., Buglyo, P., and Marmion, C.J. 2011. Suberoylanilide hydroxamic acid, a potent histonedeacetylase inhibitor: its X-ray crystal structure and solid state and solution studies of its Zn(II), Ni(II), Cu(II) and Fe(III) complexes. Journal of Inorganic Biochemistry, vol. 105. pp. 763-769. [ Links ]
Herrera, R.U. 2003. Recent developments and advances in formulations and applications of chemical reagents used in froth flotation. Mineral Processing and Extractive Metallurgy Review, vol. 24, no. 2. pp. 139-182. [ Links ]
He, X. J. and Vaisey, M. 2012. Development and research on Mt Weld rare earth ore, Australia. Proceedings of the XXVI International Mineral Processing Congress, New Delhi, India, 23-28 September. Indian Institute of Mineral Engineers. pp. 5860-5890. [ Links ]
Hu, Y.H. 2014. Mineral Flotation. Cha Sha, China (in Chinese). [ Links ]
Jiang, Y., Zhou, L., Zhou, X., and Zhao, B. 2010. Novel condensed ring carboxylic acid studied in the flotation behavior of diaspora and aluminosilicates. Separation Science and Technology, vol. 45. pp. 2475-2480. [ Links ]
Jiang, Y R., Li, W., and Feng, R. 2011. Preparation and performance of 4-alkyl- 4,4- bis(hydroxyl carbamoyl) carboxylic acid for flotation separation of diaspore against aluminosilicates. Minerals Engineering, vol. 24, no. 14. pp. 1571-1579. [ Links ]
Jordens, A., Marion, C., Kuzmina, O., and Waters, K.E. 2014. Surface chemistry considerations in the flotation of bastnasite. Minerals Engineering, vol. 66-68. pp. 119-129. [ Links ]
Jordens, A., Chen, Y.P., and Waters, K.E. 2013. A review of the beneficiation of rare earth element bearing minerals. Minerals Engineering, vol. 41. pp. 97-114. [ Links ]
Krishnamurthy, N. and Guptac, K. 2002. Rare earth metals and alloys by electrolytic methods. Mineral Processing and Extractive Metallurgy Review, vol. 22, no. 4-6. pp. 477-507. [ Links ]
Lee, J.S., Nagaraj, D.R., and Coe, J.E. 1998. Practical aspects of oxide copper recovery with alkyl hydroxamates. Minerals Engineering, vol. 11. pp. 929-939. [ Links ]
Liu, G.H., Wu, H.B., Liu, J., Shi, X.G., and Liu, Q.B. 1989. Stability constants of compound of rare earth with hydroxamic acid. Journal of Inorganic Chemistry, vol. 5, no. 2. p. 30 (in Chinese). [ Links ]
Luo, Q.H. 2012. Coordination Chemistry. Science Press, Beijing, China (in Chinese). [ Links ]
Mousumi, G. and Venugopal, R. 2016. Modeling of flotation process-an overview of different approaches. Mineral Processing and Extractive Metallurgy Review, vol. 37, no. 2. pp. 120-133. [ Links ]
O'Brien, E.C., Le, R.S., Vaillain J., Fitzgerald, D.J., and Nolan, K.B. 1997. Metal complexes of salicylhydroxamic acid and o-acetylsalicyl hydroxamic acid. Inorganica Chimica Acta, vol. 266, no, 1. pp. 117-120. [ Links ]
Pugh, R.J. 1991. The interaction of polyethylene oxide non-ionic surfactant layers adsorbed on hydrophobic minerals. International Journal of Mineral Processing,, vol. 33, no. 1-4. pp. 307-320. [ Links ]
Ren, J., Lu, S., Song, S., and Niu, J. 1997. New collector for rare earth mineral flotation. Minerals Engineering, vol. 10. pp. 1395-1404. [ Links ]
Sreenivas, T. and Padmanabhan, N. P. 2002. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids and Surfaces A, vol. 205, no. 1-2. pp. 47-59. [ Links ]
Wang, C.H., Qiu, X.Y., Hu, Z., Wang, T., and Li, H.W. 2014. Flotation mechanism of bastnaesite by salicylhydroxamic acid. Journal of the Chinese Society of Rare Earths, vol. 32, no. 6. pp. 727-733. [ Links ]
Wang, X.M., Liu, J., and Miller, J.D. 2008. Adsorption and self-assembly of octyl hydroxamicacid at a fluorite surface as revealed by sum-frequency vibrational spectroscopy. Journal of Colloid and Interface Science, vol. 325. pp. 398-403. [ Links ]
Wu, X.Q. and Zhu, J.G. 2006. Selective flotation of cassiterite with benzohydroxamic acid. Minerals Engineering, vol. 19. pp. 1410-1417. [ Links ]
Xu, H.F., Zhong, H., Tang, Q., Wang, S., Zhao, G., and Liu, G.Y. 2015. A novel collector2-ethyl-2-hexenoic hydroxamic acid: Flotation performance and adsorption mechanism to ilmenite. Applied Surface Science, vol. 353. pp. 882-889. [ Links ]
Xin, Q.Y., Pei, W.W., and Xu, R.Q. 2006. Basic Organic Chemistry. Chemical Industry Press, Beijing, China (in Chinese). [ Links ]
Xu, J.Q., Xu, X.J., and Wang, J.W. 2002. Synthesis of 1-hydroxy-2- naphthylhydroximic acid and application to collecting rare earth minerals. NonferrousMetals, vol. 54, no. 3. pp. 72-73 (in Chinese). [ Links ]
Yosry, A.A. 1990. Extraction and refining of high purity terbium metal from rare earth resources. Mineral Processing and Extractive Metallurgy Review, vol. 7, no. 2. pp. 95-114. [ Links ]
Zhou, F., Yan, C.J., Wang, H.Q., Sun, Q., And Wang, Q.Y. 2015a. Flotation behavior of four C18 hydroxamic acids as collectors of rhodochrosite. Minerals Engineering, vol. 78. pp. 15-20. [ Links ]
Zhou, F., Wang, L.X., Xu, Z.H., Liu, Q.X., and Chi, R.A. 2015b. Reactive oily bubble technology for flotation of apatite, dolomite and quartz. International Journal of Mineral Processing, vol. 134. pp. 74-81. [ Links ]
Paper received Oct. 2016
Revised paper received Apr. 2017














