Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 n.6 Johannesburg Jun. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n6a4
PAPERS OF GENERAL INTEREST
Molecular modelling of tantalum in an aqueous phase
M.J. Ungerer; C.G.C.E. van Sittert; D.J. van der Westhuizen; H.M. Krieg
Chemical Resource Beneficiation (CRB), Northwest University, Potchefstroom, South Africa
SYNOPSIS
The transition metals tantalum (Ta) and niobium (Nb) are of significant importance, for example in the nuclear energy sector where they are used as cladding materials, as well as in capacitors and specialized materials. For these applications a high-purity metal is needed. The separation of Ta and Nb is always a challenge since they are found together in nature and have similar chemical and physical properties, resulting in costly and laborious separation processes. A technology that has been used successfully for the separation of these metals entails solvent extraction (SX)1. While separation was achieved in a previous SX study using a sulphuric acid (H2SO4) medium with the extractants diiso-octyl phosphinic acid (PA) and di-(2-ethylhexyl) phosphoric acid (D2EHPA), due to the absence of speciation data for Ta and Nb it is not clear how the separation occurred.
One method that might be suitable for determining the speciation of a reaction is molecular modelling. Calculations based on the density-functional theory (DFT) are now used not only for light elements and small molecules, but also metal complexes, heavy metals, and especially metal separation in SX2. In this study the aqueous phase used during SX was investigated by studying periodic systems of Ta, as a metal and in salt form, when it is in contact with H2O and H2SO4. The results were used to predict the reaction mechanism occurring during SX. Results showed that (i) in a 1:1 acid-water ratio, the deprotonation of H2SO4 was endothermic, (ii) in a 1:5 ratio deprotonation was exothermic forming HSO4-, and (iii) in a 1:10 ratio double deprotonation occurred to form SO42- exothermically.
Keywords: tantalum, niobium, solvent extraction, reaction mechanism, molecular modelling.
Introduction
Tantalum (Ta) and niobium (Nb) are two metals found in the same group (VB) of the periodic table of elements. Owing to their similar chemical and physical properties, they are difficult to separate. Ta and Nb are usually found together in various minerals, of which the most important are columbite ((Fe, Mn, Mg)(Nb, Ta)2O6) and tantalite ((Fe, Mn)(Nb, Ta)2O6) (Agulyanski, 2004). Ta is used in a variety of applications, including capacitors in electronic circuits, rectifiers, pins for bone fixtures, surgical and dental instruments, and in chemical heat exchangers (Krebs, 2006). For many applications, pure Ta is needed; however, increasing purity entails a proportional increase in production cost. One way of ensuring an economically viable process for the production of high-purity Ta is to find a cost-effective way to separate Ta and Nb.
Solvent extraction (SX) is used for the separation and purification of various metals, including copper (Bidari, Irannejad, and Gharabaghi, 2013), nickel (Noori etal, 2014), iron (Li et al., 2011), platinum group metals (PGMs) (Kumar et al., 2008), zirconium (Biswas and Hayat, 2002), hafnium (Lee, Banda, and Min, 2015), and Ta, and Nb (Zhu and Cheng, 2011). Ungerer et al. (2014) studied the separation of Ta and Nb (in the form of MF5) by SX using safer and more environmentally friendly chemicals and techniques. Although partial separation was achieved in a sulphuric acid (H2SO4) medium with the extractants diiso-octyl phosphinic acid (PA) and di-(2-ethylhexyl) phosphoric acid (D2EHPA), the main obstacle remained the lack of data on the speciation of Ta and Nb compounds, without which it was not possible to fully explain the separation data obtained.
One method that could be used for speciation of the compounds is computational methods for SX, which entails a step-by-step analysis of the extraction process on a molecular level and determination of the molecular reactions occurring during SX from a thermodynamic perspective, which could lead to the development of a new method for the analysis of Ta and Nb separation by SX.
In this study we propose the use of molecular modelling to determine the behaviour of Ta in a sulphuric acid medium. Reactions of H2SO4 and water (Equations [1] and [2]) and TaF5 in H2SO4 (Equations [3] and [4]) were investigated.
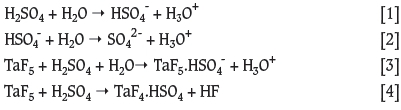
Computational methods
For the molecular modelling of H2SO4 with H2O (Equations [1] and [2]) as well as TaF5 with H2SO4 (Equations [3] and [4]), both in an aqueous phase, the DMol3 module - a density functional theory (DFT) (Hohenberg and Kohn, 1964; Levy, 1979) quantum mechanical modelling method of the Materials Studio 6.1 software from Accelrys (2012) was used. For all the calculations on the different molecules, a geometry optimization (Perdew and Wang, 1992; Delley, 1992) was first performed with the generalized gradient-corrected approximation (GGA) with Perdew-Wang (1992) correlation functional (PW91). The basis set used was DNP (double-numeric polarization functions) with basis file 4.4 and OBS dispersion correction. The core treatment parameter was set to 'All Electron' and therefore all the calculations were done for the electrons as if they are valence electrons. Under the electronic properties, smearing of 0.005 Hartree (Ha) was also chosen (Delley, 1995) and the solvation model COSMO (conductor-like screening model) (Delley, 2006) was used, with water as the solvent with a dielectric constant of 78.54. COSMO was used to account for the surrounding of implicit H2O molecules. After the geometry optimization, various properties were calculated using single-point energy calculations with the same settings as stated previously. The calculations were done in the aqueous phase at 0 K and the energy correction term was added to give Gibbs free energy values at 298.15 K.
Results and discussion
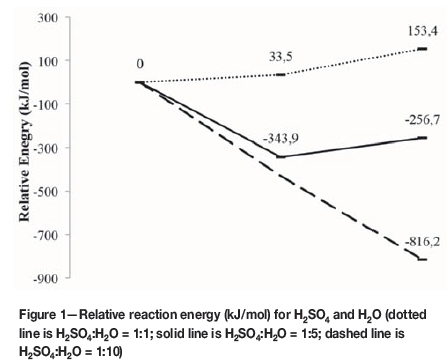
The relative energies for Reactions [1] and [2] are presented in Figure 1, where the dotted line represents the reaction energies when using a 1:1 H2SO4:H2O ratio and the solid line when using a 1:5 H2SO4:H2O ratio, where five explicit water molecules were added. For each line three points are presented, the first being the energy of the starting materials, the second the energy of the intermediate products, and the third the energy of the final products. When using a 1:1 H2SO4:H2O ratio (dotted line) it can be seen that when one H2SO4 molecule reacted consecutively with two H2O molecules to firstly form HSO4- and H3O+, and then SO42- and 2H3O+, 33.5 kJ/mol and 153.4 kJ/mol were needed respectively, indicating an endothermic reaction for both steps.
However, according to laboratory results and values from the literature, Reaction [1] is highly exothermic and Reaction [2] moderately exothermic. Steyl (2009) used DFT modelling (DMol3 v.4.2) to show that when H2SO4 reacted with H2O, five H2O molecules were needed to form an outer sphere around the H2SO4 molecule to react to form HSO4-. Therefore a balanced reaction equation was modelled where five H2O molecules reacted with H2SO4 as follows:
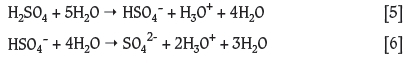
According to Figure 1 (solid line) the reaction becomes exothermic, as expected, when sufficient water molecules are available. To determine the effect of adding further water molecules it was decided to model the H2SO4-H2O system with 10 explicit waters surrounding the acid (Figure 1 -dashed line). After the geometry optimization of the 1:10 system, it was seen that double deprotonation of H2SO4 took place, without the formation of HSO4- as an intermediate step. The energy decrease to -816 kJ/mol indicates an exothermic reaction with a stabilized ion forming as the final product. As when using five H2O molecules, the surrounding H2O molecules stabilized the ion by hydrogen bonding. Ding and Laasonen (2004), using DFT modelling with PW91(DNP) as well as BLYP calculations, showed that when less than five H2O molecules are present with H2SO4 the first deprotonation occurs exothermically, and when eight to ten H2O molecules surround H2SO4, the second deprotonation also occurs, resulting in the formation of SO42- with the other surrounding H2O molecules forming hydrogen bonds and stabilizing the formed ions. Hammerich, Buch, and Mohamed (2008) used ab initio modelling methods and also showed that when ten H2O molecules are present double deprotonation occurs, but also observed proton hopping between the different oxygen sites of HSO4- and the surrounding H2O molecules. Although it is generally assumed that H2SO4:H2O is a 1:1 reaction, it was shown that, energetically, from five to ten explicit H2O molecules are needed for the reaction to occur. According to these results, the 1:10 reaction of H2SO4 in H2O showed the best correlation to the real system.
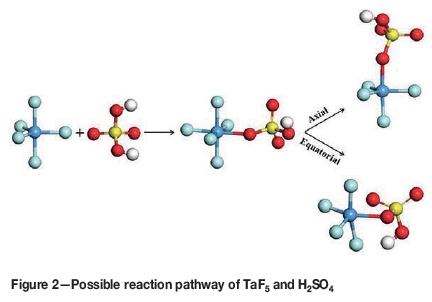
In the second part of the investigation, the reaction of TaF5 and H2SO4 in the presence of 1, 5 and 10 H2O molecules was modelled at TaF5:H2SO4:H2O ratios of 1:1:1, 1:1:5, and 1:1:10. Since TaF5 has a trigonal bipyramidal structure, the H2SO4 molecule will most likely approach from an equatorial position (where more space and best orbital overlap is available) to form TaF5.HSO4- (octahedral structure) before rearranging to TaF4.HSO4, where the HSO4- group can be either axial or equatorial (Figure 2).
As with Equations [1] and [2], it was expected that the relative reaction energies would be lower when adding explicit water molecules to Equations [3] and [4]. To confirm this, the same calculations done for Equations [1] and [2] were performed for Equations [3] and [4]. The relative energies of the reactions of TaF5 with H2SO4 and H2O are shown in Figure 3. The dotted line shows the energies for a TaF5:H2SO4:H2O ratio of 1:1:1, the solid line the energies for a TaF5:H2SO4:H2O ratio of 1:1:5, and the dashed lines the energies when the TaF5:H2SO4:H2O ratio is 1:1:10. Again, the first data point is the energy of the reagents, the second the energy of the intermediates, and the third the energy of the products.
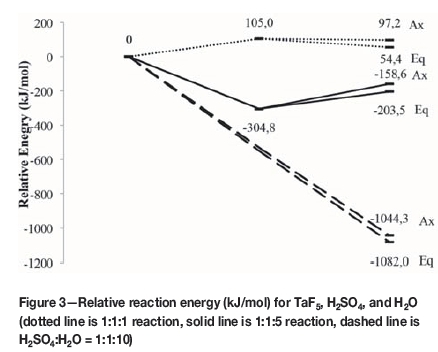
As shown in Figure 2, the position of the HSO4- group can be either axial or equatorial. In Figure 3, for the 1:1:1 reaction (dotted line) 105 kJ/mol is needed for the HSO4-group to bond to TaF5, before HF is evolved and TaF4.HSO4 is formed at 97.2 kJ/mol and 54.4 kJ/mol for the axial and equatorial positions respectively, indicating an endothermic reaction. However when using more H2O (five molecules -solid line) -304.8 kJ/mol is needed to form TaF5.HSO4-, indicating that a stable ion forms. The formation of TaF4.HSO4 in the axial position requires -158.6 kJ/mol, and in the equatorial position -203.5 kJ/mol, indicating an exothermic reaction. The same trend was observed as for the 1:1:1 reaction, where the molecule with the HSO4- group in the equatorial position was at a lower energy than when the group is axial. This is due to the orbital overlap that occurs between the HSO4- group and TaF5 when the reaction takes place. Steyl (2009) obtained similar results.
The 1:1:10 reaction of TaF5:H2SO4:H2O was also modelled. Again, the HSO4- ion bonded axially and equatorially, and as with the 1 H2SO4:10 H2O system, double deprotonation occurred. The intermediate molecule TaF4.HSO4 did not form, but the TaF4SO4- molecule formed and was stabilized by the surrounding H3O+ ions and H2O molecules. This would imply that the 1:1:10 reaction scheme had the lowest energy and would therefore be energetically the most likely.
Furthermore, it was seen from the modelling results that when the HSO4- group was in the equatorial position it formed a bidentate bond to Ta, lowering the overall energy of the molecule and changing the oxidation state of Ta from 5+ to 4+ in both the 1:5 and the 1:10 system.
Conclusion
The reactions of H2SO4 with H2O and TaF5 with H2SO4 and H2O were investigated. When modelling a 1:1 reaction of acid and water an endothermic reaction was observed. The modelling software COSMO was used to account for the surrounding implicit H2O molecules. By adding five explicit H2O molecules to the reaction, an exothermic reaction was observed for the reactions of H2SO4 with H2O. This indicated that COSMO adds a correction term for the long-range interactions that could occur if these reactions occurred in water as a medium, but does not show or calculate the explicit reactions and hydrogen bonding that occur with H2O in the short range. This hydrogen bonding stabilizes the molecules, resulting in lower energy values. With the addition of 10 explicit H2O molecules a double deprotonation was observed with the formation of SO42- stabilized by the surrounding hydrogen bonds. The same tendencies were observed for the reactions of TaF5 with H2SO4 and H2O, where the explicit H2O molecules lowered the overall reaction energies, showing an exothermic reaction. The resulting ions were stabilized with the surrounding H3O+ ions and H2O molecules.
Further investigations are needed to determine the side reactions and other geometries that may occur during these reactions, as well as the possible influence if a change in oxidation state occurs.
Acknowledgements
The author would like to thank the South African Nuclear Energy Corporation SOC Limited (Necsa) and the New Metals Development Network (NMDN) of the Advanced Metals Initiative (AMI) of the Department of Science and Technology (DST) for financial support, and the North-West University High Performance Computing (NWU-HPC) centre for the use of their facilities and their support.
References
Accelrys Software Inc. 2012. Material Studio Modelling Environment, version 6.1. San Diego. [ Links ]
Agulyanski, A. 2004. The chemistry of tantalum and niobium fluoride compounds. Elsevier, San Diego, Oxford, London. [ Links ]
Bidari, E., Irannejad, M., and Gharabaghi, M. 2013. Solvent extraction recovery and separation of cadmium and copper from sulphate solution. Journal of Environmental Chemical Engineering, vol. 1. pp. 1269-1274. [ Links ]
Biswas, R.K. and Hayat, M.A. 2002. Solvent extraction of zirconium(IV) from chloride media by D2EHPA in kerosene. Hydrometallurgy, vol. 63. pp. 149-158. [ Links ]
Delley, B. 1992. Ground-state enthalpies: evaluation of electronic structure approaches with emphasis on the density functional method. Journal of Physical Chemistry A, vol. 110. pp. 13632-13639. [ Links ]
Delley, B., and Scherrer, P. 1995. DMol, a standard tool for density functional calculations: review and advances Modern Density Functional Theory: a Toolf or Chemistry. Seminario, J.M. and Politzer, P. (eds). Theoretical and Computational Chemistry Series, vol. 2. Elsevier. [ Links ]
Delley, B. 2006. The conductor-like screening model for polymers and surfaces. Molecular Simulation, vol. 32. pp. 117-123. [ Links ]
Ding, C.G., and Laasonen, K. 2004. Partially and fully deprotonated sulfuric acid in H2SO4(H2O)n (n = 6 - 9) clusters. Chemical Physics Letters, vol. 390. pp. 307-313. [ Links ]
Hammerich, A.D., Buch, v., and Mohamed, F. 2008. Ab initio simulations of sulfuric acid solutions. Chemical Physics Letters, vol. 460. pp. 423-431. [ Links ]
Hohenberg, P. and Kohn, W. 1964. Inhomogeneous electron gas. Physical Review B, vol. 136. p. B864. [ Links ]
Krebs, R.E. 2006. The History and Use of Our Earth's Chemical Elements: A Reference Guide. 2nd edn. Greenwood Press, Westport, USA. [ Links ]
Kumar, J.R., Lee, H.I., Lee, J.Y., Kim, J.S., and Sohn, J.S. 2008. Comparison of liquid-liquid extraction studies on platinum(IV) from acidic solutions using bis(2,4,4-trimethylpentyl) monothiophosphinic acid. Separation and Purification Technology, vol. 63. pp. 184-190. [ Links ]
Lee, M.S., Banda, R., and Min, S.H. 2015. Separation of Hf(IV)-Zr(IV) in H2SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations. Hydrometallurgy, vol. 152. pp. 84-90. [ Links ]
Levy, M. 1979. Universal variational functionals of electron densities, first- order density matrices, and natural spin-orbitals ans solution of the v- representability problem. Proceedings of the National Academy of Sciences, vol. 76. pp. 6062-6065. [ Links ]
Li, X., Wei, C., Deng, Z., Li, M., Li, C., and Fan, G. 2011. Selective solvent extraction of vanadium over iron from a stone coal/black shale acid leach solution by D2EHPA/TBP. Hydrometallurgy, vol. 105. pp. 359-363. [ Links ]
Narbutt, J. and Czerwinski, M. 1992. Computational chemistry in modelling solvent extraction of metal ions. Solvent Extraction Principles and Practice. Rydberg, J., Cox, M., Musikas, C., and Choppin, G.R. (eds.). Wiley, New York. Chapter 16. [ Links ]
Noori, M., Rashchi, F., Babakhani, A., and Vahidi, E. 2014. Selective recovery and separation of nickel and vanadium in sulfate media using mixtures of D2EHPA and Cyanex 272. Separation and Purification Technology, vol. 136. pp. 265-273. [ Links ]
Perdew, J.P., and Wang, Y. 1992. Accurate and simple analytic representation of the electron-gas correlation energy. Physical Review B, vol. 45. pp. 13244-13249. [ Links ]
Steyl, J.D.T. 2009. Kinetic modelling of chemical processes in acid solution at ts 200°C. (i) thermodynamics and speciation in H2SO4-metal (ii) SO4-H2O system. Proceedings of Hydrometallurgy 2009, Misty Hills, Muldersdrift, Gauteng 24-26 February. Southern African institute of Mining and Metallurgy, Johannesburg. pp. 401-444. [ Links ]
Ungerer, M.J., van der Westhuizen, D.J., Lachmann, G., and Krieg, H.M. 2014. Comparison of extractants for the separation of TaF5 and NbF5 in different acidic media. Hydrometallurgy, vol. 144-145. pp. 195-206. [ Links ]
Zhu, Z. and Cheng, C.Y. 2011. Solvent extraction technology for the separation and purification of niobium and tantalum: a review. Hydrometallurgy, vol. 108. pp. 1-12. [ Links ]
Paper received Apr. 2016
Revised paper received Sept. 2016
1 Narbutt, J. and Czerwinski, M. 1992. Computational chemistry in modelling solvent extraction of metal ions. Solvent Extraction Principles and Practice. Rydberg, J., Cox, M., Musikas, C., and Choppin, G.R. (eds). Wiley, New York. Chapter 16.
2 Ungerer, M.J., van der Westhuizen, D.J., Lachmann, G., and Krieg, H.M. 2014. Comparison of extractants for the separation of TaF5 and NbF5 in different acidic media. Hydrometallurgy, vol. 144-145. pp. 195-206.














