Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 n.4 Johannesburg Apr. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n4a10
GENERAL PAPERS
Potential of biotechnology for metals extraction in Zimbabwe: a review
W. ChingwaruI, II; J. VidmarII, III; C. ChingwaruI
IDepartment of Biological Sciences, Faculty of Science, Bindura University Science Education, Bindura, Zimbabwe
IIMaribor Institute of Biomedical Sciences, Maribor, Slovenia
IIIInstitute Ceres/Zavod Ceres, Celje, Slovenia
IVDepartment of Plastic and Reconstructive Surgery, University Medical Centre Maribor, Maribor, Slovenia
SYNOPSIS
Zimbabwe is endowed with rich deposits of minerals such as diamonds, platinum, coal, uranium, lithium, gold, antimony, iron, and chrome. Bioleaching has been implemented as an efficient and low-cost method to extract metals such as copper, cobalt, and gold from sulphide and/or iron-containing ores and mineral concentrates in a number of countries around the globe. Zimbabwe, despite being a world leader in mineral wealth, has gone through years of economic stagnation which have brought with them energy shortages. Bioleaching is an innovative way to recover minerals from ores using relatively low-capital-cost and non-polluting technology. Principally, iron- and sulphur-oxidizing bacteria can be used to oxidize iron and sulphide to ferric iron and sulphuric acid, respectively, and the ferric iron oxidizes the insoluble metal sulphides to soluble metal sulphates that can be readily recovered from solution. Although some minerals such as gold are inert to biological reactions, they can be liberated using bacteria that act on certain types of ores and other minerals that co-occur with these minerals. The geology of the mineralized areas in Zimbabwe, rich in chalcopyrite / pyrite, allows a number of microorganisms to be used for the extraction of minerals by bio-oxidation. This paper reviews the potential of bioleaching in the country.
Keywords: bioleaching, bio-oxidation, gold, nickel, copper.
Introduction
Zimbabwe has rich deposits of minerals including diamonds, platinum, coal, uranium, lithium, gold, antimony, iron, and chrome. Bioleaching is defined as the use of microorganisms to facilitate the extraction of metals from sulphide or iron-containing ores or concentrates (Rawlings, 2004, 1997). Bioleaching has been shown to be an efficient (Blowes, Ptacek, and Jurjovec, 2003; Nordstrom and Southam, 1997; Schippers, 2004) and low-cost (Schippers et al., 2010) method to extract minerals in many countries around the globe. For example, aerobic Fe(II)-and sulphur-oxidizing bacteria have been shown to be up to two orders of magnitude more efficient in the oxidation of pyrite than the chemical processes (Blowes, Ptacek, and Jurjovec, 2003; Nordstrom and Southam, 1997; Schippers, 2004). Additionally, bioleaching can be used to extract minerals from low-grade ores, sulphidic waste rock dumps, and tailings efficiently (Schippers et al., 2010).
The process of bioleaching has been shown to occur largely by the indirect contact between microorganisms and ferric iron, the latter acting as an intermediate electron acceptor. 'Direct leaching' was believed to involve intimate microbial and mineral contact with direct enzymatic oxidation of the mineral under aerobic conditions (Berry and Murr, 1976; Bennet and Tributsch, 1978). However, the concept of direct leaching was disproved (Fowler and Crundwell, 1999). Bacterial leaching is believed to occur when bacteria attach to and accelerate the reaction of some minerals through the removal of sulphur, which would constitute a diffusion barrier in chemical leaching (Fowler and Crundwell, 1999). On the other hand, bacterial leaching can occur as a result of pH modifications at the mineral surface through bacterial action. The rate of dissolution of pyrite was shown to increase as a result of changes in the concentrations of ferric and ferrous ions and in pH due to bacterial action at mineral surfaces (Fowler and Crundwell, 1999; Zeng, Schumann, and Smart, 2013). The contact mechanism considers that bioleaching occurs through activities of the extracellular polymeric substances that are released into a mineral-bacteria interphase in biofilms that are formed when bacterial cells attach to the surfaces of sulphide minerals (Bobadilla Fazzini, Levican, and Parada, 2011; Sand and Gehrke, 2006). The oxidation of elemental sulphur by acidithiobacilli relies on oxygen as electron acceptor, which assists the solubilization of metal sulphides present in the mineral surface (Rohwerder et al., 2003). Pseudomonas fluoresceins, uses nitrate instead of oxygen as a final electron acceptor during anaerobic respiration (Samuelsson, Cadez, and Gustafsson, 1988), yielding cyanide which leaches minerals such as silver, gold, and platinum (Brandl et al., 2008; Pradhan and Kumar, 2012).
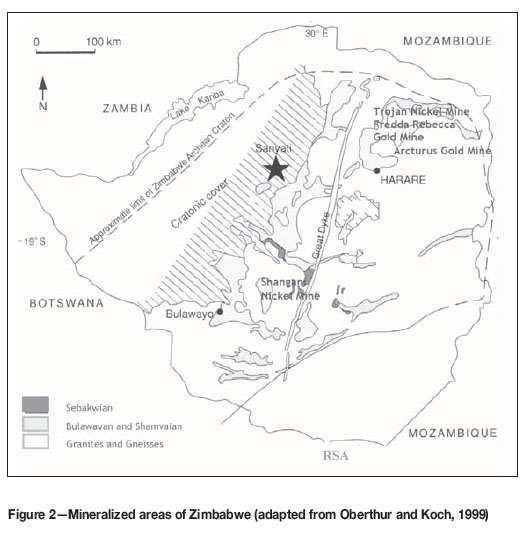
The capital cost of bioleaching is widely claimed to be considerably lower than that of conventional smelting (Dresher, 2004; Mintek, n.d.). No meaningful indications of the actual savings were found at this stage. Despite the demonstration of higher extraction efficiency and greater cost-effectiveness of the bioleaching process, its potential in the Zimbabwean geological and economic contexts has not been explored. Zimbabwe is going through economic challenges that affect its capacity to exploit its mineral wealth. The Zimbabwean mining sector continues to rely on energy- and cost-intensive mineral extraction methods, including smelting and chemical leaching. The geological formations that make up the principal mining areas of the country, concentrated along the Great Dyke, are rich in minerals that include platinum, uranium, lithium, gold, antimony, iron, and chrome. The rock types including orthopyroxenite, olivine basalt, gabbro, coarse websterite, fine websterite and olivine gabbro (Mukasa, Wilson, and Carlson, 1998; Oberthur et al., 2013). Of these, gold, base metal sulphides (including iron), and primary uranium are bioleachable (Johnson, Kanao, and Hedrich, 2012; Pal et al., 2010). The country's mineralization is divided into zones, namely the Sebakwean, Bulawayan, and Shamvaian, and granites and gneisses (Figure 2).

Potential for bioleaching in Zimbabwe
The Great Dyke
The Great Dyke (actually not strictly a dyke) is a linear lopolithic geological feature that spans approximately 550 km from its northern end to its southern end (Hughes, 1970) (Figure 2). The Great Dyke is rich in ore deposits, including gold, silver, chromium, platinum, nickel, and asbestos (Guilbert and Park, 1986). The Great Dyke consists of a group of layered ultramafic intrusions that are locally overlain by erosional remnants of gabbroic rock (Mukasa, Wilson, and Carlson, 1998). The sequence includes orthopy-roxenite, olivine basalt, gabbro, coarse websterite, fine websterite, and olivine gabbro (Mukasa, Wilson, and Carlson, 1998) and chalcopyrite-bearing rocks (Li et al., 2007). The Main Sulphide Zone of the Great Dyke contains platinum-group element (Pt and Pd) sulphides, arsenides, tellurides, and base metal sulphide assemblages (Li et al., 2007). A number of microorganisms including Ramlibacter tataouinensis, Acidithiobacillus thiooxidans (Bobadilla et al., 2011), Acidithiobacillus flerrooxidans (Karavaiko et al., 1986), Acidithiobacillus caldus (Watling et al., 2015), Acidiphilum species (spp.) (Parada et al.), Leptospirillum ferriphilum (Fu et al., 2008), Sulfobacillus thermosufi-dooxidans (Stott et al., 2000), Ferroplasma thermophilum, (Zhou et al., 2008) and Aspergillus niger (Aung et al., 2005) have been shown to have great potential to leach the minerals that are found in the country.
Bioleaching of gold
Gold deposits (Figure 2). In arsenic-rich gold concentrates, the gold particles are enclosed in sulphide minerals, including arsenopyrite (FeAsS), pyrite (FeS2), realgar (As2S2), or orpiment (As2S3) (Xie et al., 2013). In order to obtain satisfactory gold recoveries, oxidative pretreatment is required to break down the sulphide minerals (Xie et al., 2013). Gold is finely disseminated in refractory sulphide ores, which reduces the capacity of conventional cyanidation to recover gold (Lindstrom, Gunneriusson, and Tuovinen, 1992). In the bioleaching process, bacteria break down the sulphide minerals by oxidative dissolution, thus dissolving ferrous iron (Fe2+) and exposing gold to the cyanide solution (Lindstrom, Gunneriusson, and Tuovinen, 1992). Biooxidation offers a cost-effective and efficient way to extract gold from refractory ores (Schippers et al., 2014). A number of pilot studies have demonstrated that biooxidation can be used in the extraction of gold (Brierley, 1997; Gericke, Neale, and van Staden, 2009; Karavaiko et al., 1986; Marchant, 1986). Marchant (1986) demonstrated a 75% increase in gold recovery with partial bacterial oxidation of sulphide minerals, particularly arsenopyrite. A pilot study in the former USSR by Karavaiko et al., (1986) demonstrated 90% gold recovery through the oxidation of arsenopyrite by A.ferrooxidans. The first commercial biohydrometallurgical process to extract gold in the world, patented as the Biox® process and involving the pretreatment of refractory gold-bearing arsenopyrite, was developed by Gencor SA Ltd (now BHP Billiton) at Fairview mine in South Africa (Brierley, 1997). The Biox® process has been shown to increase gold recovery by up to 95% (Brierley, 1997), depending on the mineralogy and gold deportment of each ore. Another biohydrometallurgical process, which operates at temperatures higher than those in the Biox® process, was developed and commercialized by Australian-based company BacTech (Brierley, 1997). Bactech, working in partnership with Mintek, established a Bacox™ bioleaching plant, in Tasmania (Gericke, Neale, and van Staden, 2009). In 2001, Bactech and Mintek established another bioleaching plant at the BioGold toll treatment facility located near the city of Laizhou, in the Shandong Province of PR China. Both of these plants remain in operation to date. (Gericke, Neale, and van Staden, 2009). Both the Biox® and BacoxTM processes are carried out in aerated stirred-tank reactors (Brierley, 1997), which are an alternative to heap leaching and Geobiotics processes. Biooxidation heap leaching is used by the Newmont Gold Company in Nevada, USA. Geobiotics Inc. built a demonstration facility in South Dakota, USA, which was based on the use of heaps of supporting rocks coated with an ore concentrate (Morin, 1995). Biooxidation of ore using the Geobiotics technology was shown to increase gold recovery by between 55% and 74% (Morin, 1995). However, the recovery of metals through the biooxidation of whole ores results in marginal economics (Gericke, Neale, and van Staden, 2009; Whitlock, 1997).
The Mazowe gold area
Gold mineralization in the Mazowe area, approximately 40 northwest of Harare, is hosted in a variety of lithologies of the Archean Harare-Bindura-Shamva greenstone belt (Figure 2) (Oberthur et al., 2000). The Mazowe gold mines are based on pyrite-rich reefs, the Bernheim group of mines are hosted on arsenopyrite-rich metabasalt ores, and Stori's Golden Shaft and the Alice mine are hosted in metabasalts containing sulphide-poor quartz veins (Oberthur et al., 2000). Notably, the sulphide-rich ores in Stori's Golden Shaft mine are characteristically low in gold content (Oberthur et al., 2000). Biooxidation may not be a viable option for the Stori ores due to the low sulphide content of the metabasalts and low gold concentrations in certain places. The bioextraction of gold from the pyrite-rich reefs in the Mazowe gold mines and Bernheim group of mines may be driven by bacteria that include Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, Acidithiobacillus caldus, Acidiphilium cryptum, Leptospirillumferriphilum, Sulfobacillus thermosulfdooxidans, Ferroplasma thermophilum, and Sulfolobus spp. The Biox® and Bacox™ processes may be viable technologies for the bioextraction of gold at the Mazowe gold mines.
The Arcturus-Harare gold area
The Arcturus gold mine, located within the Shamvaian Group (Figure 2), is hosted in the Iron Mask Formation, which comprises a succession of metamorphosed felsic volcanics and associated metasediments including prominent bands of sulphide-facies iron formation, phyllites, meta-arenites, and quartzose schists (Oberthur et al., 2000). The mineralized shear zones in the Arcturus mine are characterized by silicifi-cation, biotite, and K-feldspar alteration. Gold mineralization is associated with pyrrhotite, arsenopyrite, and also occurs as free gold, which contributed up to 10% of production (http://metcorp.co.uk/operations/arcturus-mine.aspx). Biooxidation of the Arcturus ores may be possible using A. ferrooxidans, which reportedly oxidizes biotite (Bhatti et al., 2011). Furthermore, the presence of sulphide-rich iron formation may allow for bioextraction of gold-bearing ores using Acidithiobacillus ferrooxidans, which is shown elsewhere to have this capacity (Dew, Lawson, and Broadhurst, 1998; Natarajan and Iwasaki, 1983). However, silica in the Arcturus ores may be a limiting factor due to the encapsulation of gold in silica, which locally inhibits biooxi-dation (Sherlock, 2010).
Shangani and Trojan nickel mines
Bindura Nickel Corporation (BNC) owns the Shangani and Trojan nickel mines, which are located within the Archaean greenstone belts in the Bulawayan and Shamvaian groups. Ore is processed at the Trojan concentrator, resulting in a nickel-in-concentrate recovery of approximately 71%, The company's Bindura Smelter and Refinery (BSR) complex, which is being re-opened, will produce high-quality nickel cathodes, copper sulphide, and cobalt hydroxide (ASA Resource Group, 2016). Generally BNC has recorded a nickel recovery capacity of between 70.7 and 88.8% (ASA Resource Group, 2016). The two nickel orebodies are rich in pyrite, and the biooxidation process could be adapted to recover nickel. Nickel sulphide heap bioleaching has been piloted at several sites, including the University of Cape Town (Rawlings, 2005). The successful implementation of bioleaching technologies for the extraction of nickel has been demonstrated by a cooperative agreement reached between Amsterdam-headquartered talc producer Mondo Minerals and South Africa's state-owned mineral technology research council Mintek, which has seen the construction of a nickel sulphide bioleach plant at Mondo's Vuonos talc processing site in Finland (Creamer Media, January 2015 ). Microorganisms such as Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, Acidithiobacillus caldus, Acidiphilium cryptum, Leptospirillumferriphilum, Sufobacillus thermosufdooxidans, Ferroplasma thermophilum and Sulfolobus spp. can facilitate the recovery of nickel.
Iron ore at Redcliff near Kwekwe
The area around Redcliff, the main iron-bearing area in Zimbabwe, is underlain by Archaean rocks of the basement complex (Geological Society of Zimbabwe, 2014). The Archaean rock formations, primarily a volcano-sedimentary pile, are flanked by granitic rocks of the Rhodesdale batholiths in the east and the Shangani batholiths in the west (Geological Society of Zimbabwe, 2014). The stratigraphy of the greenstone belt in the Redcliff area is divided into the Sebakwean, Bulawayan, and Shamvaian groups (Geological Society of Zimbabwe, 2014). The extraction of iron from these formations was primarily through smelting in energy-intensive blast furnaces (Geological Society of Zimbabwe, 2014). Energy shortages and economic challenges in Zimbabwe in recent years have resulted in the shutdown of operations.
Acidithiobacillus ferrooxidans reportedly increases the rate of the oxidation of ferrous ions by a factor of 106 (Singer and Stumm, 1970). A. ferrooxidans and L. ferriphilum can oxidize both sulphide and ferrous iron oxidation (using O2 as a terminal electron acceptor) and ferric iron reduction (using reduced inorganic compounds like sulphur or hydrogen as electron donors), which allows them to grow under aerobic and anaerobic conditions (Ohmura et al., 2002; Pronk et al., 1992) (Figure 3). Hu et al. (2012) showed that Fe3+ ions are important for bioleaching of chalcopyrite. The presence of the bacterium can significantly increase the rate of sulphide leaching in the iron-bearing ores, thereby increasing the recovery of iron. It would be theoretically possible to extract iron using microorganisms such as A. ferrooxidans.
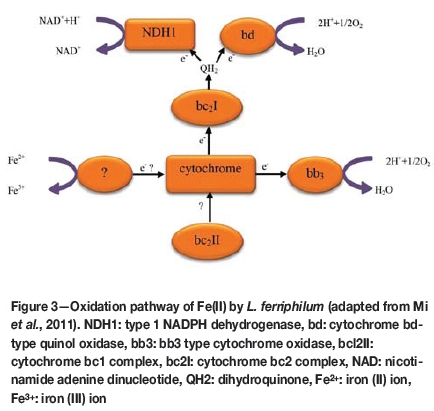
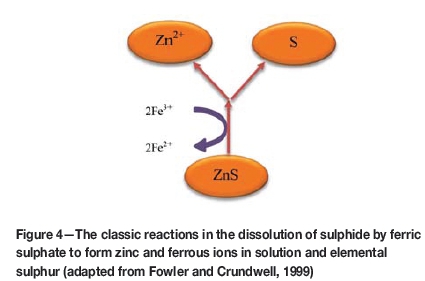
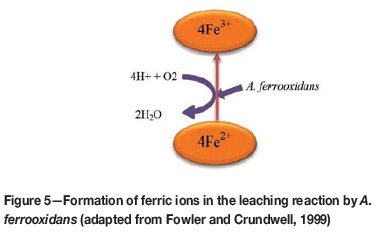
Despite this theoretical possibility, the use of this technology is limited since the solubilized iron precipitates as iron oxy-hydroxides / goethite (FeOOH) or equivalents (Parker, 2008)-the latter would require removal by smelting. Unless other biological technologies to leach iron can be developed, the use of iron bioleaching remains limited. Further research on this possibility is required.
Sanyati copper mine
The Sanyati orebody, located approximately 200 km west of Harare, is polymetallic and sulphidic (Oberthur and Koch, 1999) (Figure 2). The mineralization comprises zinc, copper, and lead sulphides (chalcopyrite, sphalerite, and galena) as well as pyrite, pyrrhotite, and arsenopyrite (Oberthur and Koch, 1999). A recent evaluation programme demonstrated that the Sanyati ore contains approximately 1.1% copper and 1.2% zinc (Oberthur and Koch, 1999). The Sanyati mine, recently opened by the Government of Zimbabwe, has capacity to produce at least 5000 tonnes of copper per annum using open pit mining, with a capacity of up to 40 kt of ore per month (Oberthur and Koch, 1999 ). Copper is extracted using solvent extraction and electrowinning (SX/EW), and the recovery of other minerals such as zinc, manganese and cobalt from the ore has been investigated (Oberthur and Koch, 1999). Bioextraction is seen as a complementary method to enhance the extraction of copper, zinc, and manganese from the Sanyati ore reserves. The sulphide-rich ores that are found in the Shamvaian Group, including Sanyati, are known to be readily oxidized by microorganisms such as A.ferrooxidans and Sufolobus species to yield copper (Dew, Lawson, and Broadhurst, 1998; Natarajan and Iwasaki, 1983; Xia et al., 2010 ) and cobalt (Dew, Lawson, and Broadhurst, 1998; Natarajan and Iwasaki, 1983).
Summary
Notwithstanding the fact that the species or strains of microorganisms reported herein are not metal-specific, their use may be convenient since the microorganisms are widely available. Given the geology of the mineralized areas of Zimbabwe and the availability of microorganisms with the capacity to extract metals through the oxidation of the ores containing minerals of interest, bioleaching could be a viable alternative to the energy- and cost-intensive extraction methods currently in use. Bioleaching can be used to facilitate the extraction of gold from the Mazowe gold district, including ores at Arcturus mine and Freda Rebecca gold mine, using such microorganisms as A. thiooxidans, A. ferrooxidans, A. caldus, A. cryptum, L. ferriphilum, S. thermosulfidooxidans, F. thermophilum, and Sulfolobus spp. Furthermore, methods such as the Biox® and BacoxTM processes could be viable, efficient, and cost-effective options for the bioextraction of gold in the Mazowe gold mines. The extraction of nickel at Shangani and Trojan nickel mines can also be enhanced through the use of such microorganisms such as A.ferrooxidans and A. caldus, which are known to oxidize the sulphide-rich ores that are found in the two mining areas. The potential of bioleaching to recover iron is not well established, however microorganisms such as A. ferrooxidans reportedly increase the rate of oxidation of ferrous ions by a factor of 106 (Singer and Stumm, 1970). The potential of bioleaching, relying primarily on A. ferrooxidans (Fowler and Crundwell, 1999), can be tapped to extract copper from ores at Sanyati copper mine. Bioleaching therefore has great potential for use as complementary technology to the conventional methods in the extraction of Zimbabwe's vast mineral wealth.
References
ASA Resource Group. 2016. Bindura Nickel Corp (BNC) www.asaukplc.com/operations-and-exploration/zimbabwe/bindura-nickel-corp-bnc.html. [Accessed 20 February 2017]. [ Links ]
Aung, K.M.M. and Ting, Y.P. 2005. Bioleaching of spent fluid catalytic cracking catalyst using Aspergillus niger. Journal of biotechnology, vol. 116, no. 2. pp.159-170. [ Links ]
Bennet, J.C. and Tributsch, H. 1978. Bacterial leaching patterns on pyrite crystal surface. Journal of Bacteriology, vol. 134. pp. 310-317. [ Links ]
Berry, V.K. and Murr, L.E. 1976. Bacterial attachment to molybdenite: An electron microscope study. Metallurgical and Materials Transactions B, vol. 6, no. 3. pp. 488-490. [ Links ]
Bhatti, T.M., Bigham, J.M., Vuorinen, A., and Tuovinen, O.H. 2011. Weathering of biotite in Acidithiobacillusferrooxidans cultures. Geomicrobiology Journal, vol. 28, no. 2. pp. 130-134. [ Links ]
Blowes, D.W., Ptacek, C.J., and Jurjovec, J. 2003. Mill tailings: hydrogeology and geochemistry. Environmental Aspects of Mine Wastes. Jambor, J.L., Blowes, D.W., and Ritchie, A.I.M. (eds). Short Course Series, vol. 31. Mineralogical Association of Canada, Vancouver, BC. pp. 95-116. [ Links ]
Bobadilla Fazzini, R.A., Levican, G., and Parada, P. 2011. Acidithiobacillus thiooxidans secretome containing a newly described lipoprotein Licanantase enhances chalcopyrite bioleaching rate. Applied Microbiology and Biotechnology, vol. 89, no. 3. pp. 771-780. [ Links ]
Brandl, H., Lehmann, S., Faramarzi, M.A., and Martinelli, D. 2008. Biomobilization of silver, gold, and platinum from solid waste materials by HCN-forming microorganisms. Hydrometallurgy, vol. 94, no. 1. pp. 14-17. [ Links ]
Brierley, J.A. 1997. Heap leaching of gold-bearing deposits: theory and operational description. Biomining. Rawlings, D.E. and Johnson, D.B. (eds). Springer, Berlin/Heidelberg. pp. 103-115. [ Links ]
Dew, D.W., Lawson, E.N., and Broadhurst, J.L. 1998. The BIOX® process for biooxidation of gold-bearing ore or concentrates. Biomining: Theory, Microbes and Industrial Processes. Rawlings, D.E. (ed.). Springer-Verlag, Berlin, Germany. pp. 45-80. [ Links ]
Dresher, W.H. 2004. Producing copper nature's way: bioleaching. CWD: Innovations, May. p. 10. [ Links ]
Fowler, T.A. and Crundwell, F.K. 1999. Leaching of zinc sulfide by Thiobacillusferrooxidans: bacterial oxidation of the sulfur product layer increases the rate of zinc sulfide dissolution at high concentrations of ferrous ions. Applied Environmental Microbiology, vol. 65, no. 12. pp. 5285-5292. [ Links ]
Fu, B., Zhou, H., Zhang, R., and Qiu, G. 2008. Bioleaching of chalcopyrite by pure and mixed cultures of Acidithiobacillus spp. and Leptospirillum ferriphilum. International Biodeterioration & Biodegradation, vol. 62, no. 2. pp. 109-115. [ Links ]
Geological Society of Zimbabwe. 2014. Tour of iron ore deposits in the Redcliff area, http://www.geologicalsociety.org.zw/sites/default/files/atlas-attachments/Tour%20of%20Redcliff%20Iron%20Ore%20Deposits%202014-10-26-compressed_4.pdf [Accessed 31 May 2015]. [ Links ]
Gericke, M., Neale, J.W., and van Staden, P.J. 2009. A Mintek perspective of the past 25 years in minerals bioleaching. Journal of the Southern African Institute of Mining and Metallurgy, vol. 109, no. 10. pp. 567-585. [ Links ]
Guilbert, J.M. and Park, C.F. Jr. 1986. The Geology of Ore Deposits. Freeman. [ Links ]
Hu, K.T., Gu, G.H., Li, S.K., and Qiu, G.Z. 2012. Bioleaching of chalcopyrite by Leptospirillumferriphilum. Journal of Central South University, vol. 19, no. 6. pp. 1718-1723. [ Links ]
Johnson, D.B., Kanao, T., and Hedrich, S. 2012. Redox transformations of iron at extremely low pH: fundamental and applied aspects. Frontiers in Microbiology, vol. 3. p. 96. DOI: 10.3389/fhlicb.2012.00096 [ Links ]
Karavaiko, G.I., Chuchalin, L.K., Pivovarova, T.A., Yemel'Yanov, B.A., and Dorofeyev, A.G. 1986. Microbiological leaching of metals from arsenopyrite containing concentrates. Fundamental and Applied Biohydrometallurgy, Process Metallurgy. Lawrence, R.W., Branion, R.M., and Ebner, H.G. Elsevier, Dordrecht. pp. 115-126. [ Links ]
Li, C., Ripley, E.M., Oberthur, T., Miller, Jr, J.D., and Joslin, G.D. 2007. Textural, mineralogical and stable isotope studies of hydrothermal alteration in the main sulphide zone of the Great Dyke, Zimbabwe and the precious metals zone of the Sonju Lake Intrusion, Minnesota, USA. Mineralium Deposita, vol. 43, no. 1. pp. 97-110. [ Links ]
Lindstrom, E.B., Gunneriusson, E., and Tuovinen, O.H. 1992. Bacterial oxidation of refractory sulfide ores for gold recovery. Critical Reviews in Biotechnology, vol. 12, no. 1-2. pp. 133-155. [ Links ]
Marchant, P.B. 1985. Commercial piloting and the economic feasibility of plant scale continuous biological tank leaching at Equity Silver Mines Limited. Fundamental and Applied Biohydrometallurgy: Proceedings of the 6th international Symposium on Biohydrometallurgy. Lawrence, R.W., Branion, R.M.R., and Ebner, H.G. (eds). Elsevier, New York. pp. 53-76. [ Links ]
Mi, S., Song, J., Lin, J., Che, Y., Zheng, H., and Lin, J. 2011. Complete genome of Leptospirillum ferriphilum ML-04 provides insight into its physiology and environmental adaptation. Journal of Microbiology, vol. 49, no. 6. pp. 890-901. [ Links ]
Mintek. Not dated. Agitated tank bioleaching. http://www.mintek.co.za/technical-divisions/biotechnology-bio/services-facilities/agitated-tank-bioleaching/ [Accessed 1 April 2016]. [ Links ]
Morin, D. 1995. Bacterial leaching of refractory gold sulphide ores. Bioextraction and Biodeterioration of Metals. Gaylarde, CC. and Videla, H.A. (eds). Cambridge University Press. p. 50. [ Links ]
Mukasa, S.B., Wilson, A.H., and Carlson, R.W. 1998. A multielement geochronologic study of the Great Dyke, Zimbabwe: significance of the robust and reset ages. Earth and Planetary Science Letters, vol. 164, no. 1. pp. 353-369. [ Links ]
Natarajan, K.A. and Iwasaki, I.1983. Role of galvanic interactions in the bioleaching of Duluth gabbro copper-nickel sulfides. Separation Science and Technology, vol. 18, no. 12-13. pp. 1095-1111. [ Links ]
Nordstrom, D.K. and Southam, G. 1997. Geomicrobiology of sulfide mineral oxidation. Geomicrobiology: Interactions Between Microbes and Minerals. Banfield, J.F. and Nealson, K.H. (eds). Reviews in Mineralogy, vol 35. Mineralogical Society of America, Washington D.C. pp. 361-390. [ Links ]
Oberthur, T., Melcher, F., Buchholz, P., and Locmelis, M. 2013. The oxidized ores of the Main Sulphide Zone, Great Dyke, Zimbabwe: turning resources into mineable reserves-mineralogy is the key. Journal of the Southern African Institute of Mining and Metallurgy, vol. 113, no. 3. pp. 191-209. [ Links ]
Oberthur, T., and Koch, H.P. 1999. The Sanyati Ore Deposits in Zimbabwe, Ore mineralogy and some geochemical considerations on supergene element redistribution. Zeitschriftfür Angewandte Geologie, vol. 45. https://www.researchgate.net/profile/Thomas_Oberthuer/publication/236354010_ The_Sanyati_ore_deposits_in_Zimbabwe_-_Introduction/links/00b49517e7395c8e34000000.pdf [Accessed 30 May 2015]. [ Links ]
Oberthur, T., Blenkinsop, T.G., Hein, U.F., Hoppner, M., Hohndorf, A., and Weiser, T.W. 2000. Gold mineralization in the Mazowe area, Harare- Bindura-Shamva greenstone belt, Zimbabwe: ii. Genetic relationships deduced from mineralogical, fluid inclusion and stable isotope studies, and the Sm-Nd isotopic composition of scheelites. Mineralium Deposita, vol. 35, no. 2-3. pp. 38-156. [ Links ]
Ohmura, N., Sasaki, K., Matsumoto, N., and Saiki, H. 2002. Anaerobic respiration using Fe(3+), S(0), and H(2) in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans. Journal of Bacteriology, vol. 184, no. 8. pp. 2081-2087. [ Links ]
Pal, S., Pradhan, D., Das, T., Sukla, L.B., and Chaudhury, G.R. 2010. Bioleaching of low-grade uranium ore using Acidithiobacillus ferrooxidans. Indian Journal of Microbiology, vol. 50, no. 1. pp. 70-75. [ Links ]
Parada, P. and Bobadilla Fazzini, R. Bioleaching of Minerals by Acidophile Microorganisms. Encyclopedia of industrial Biotechnology. [ Links ]
Parker, A.D. 2008. Oxidative dissolution of chalcopyrite in ferric media: an X- ray photoelectron spectroscopy study. PhD thesis, Curtin University of Technology. [ Links ]
Pradhan, J.K. and Kumar, S. 2012. Metals bioleaching from electronic waste by Chromo bacterium violaceum and Pseudomonads sp. Waste Management Research, vol. 30, no. 11. pp. 1151-1159. [ Links ]
Pronk, J.T., de Bruyn, J.C., Bos, P., and Kuenen, J.G. 1992. Anaerobic growth of Thiobacillusf errooxidans. Applied Environmental Microbiology, vol. 58, no. 7. pp. 2227-2230. [ Links ]
Rawlings, D.E. 1997. Biomining: Theory, Microbes and Industrial Processes. Springer-verlag, Berlin. [ Links ]
Rawlings, D.E. 2004. Microbially assisted dissolution of minerals and its use in the mining industry. Pure and Applied Chemistry, vol. 76, no. 4. pp. 847-859. [ Links ]
Rawlings, D.E. 2005. Characteristics and adaptability of iron- and suliur- oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microbial Cell Factories, vol. 4, no. 1. p. 13. http://hdl.handle.net/10019.1/5085 [ Links ]
Rohwerder, T., Gehrke, T., Kinzler, K., and Sand, W. 2003. Bioleaching review part A: progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation. Applied Microbiology and Biotechnology, vol. 63, no. 3. pp. 239-248. [ Links ]
Samuelsson, M.O., Cadez, P., and Gustafsson, L. 1988. Heat production by the denitrifying bacterium Pseudomonas fluorescens and the dissimilatory ammonium-producing bacterium Pseudomonas putrfaciens during anaerobic growth with nitrate as the electron acceptor. Applied Environmental Microbiology, vol. 54, no. 9. pp. 2220-2225. [ Links ]
Sand, W. and Gehrke, T. 2006. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron (III) ions and acidophilic bacteria. Research in Microbiology, vol. 157, no. 1. pp. 49-56. [ Links ]
Schippers, A., Breuker, A., Blazejak, A., Bosecker, K., Kock, D., and Wright, T.L. 2010. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria, Hydrometallurgy, vol. 104, no. 3-4. pp. 342-350. [ Links ]
Schippers, A., Hedrich, S., Vasters, J., Drobe, M., Sand, W., and Willscher, S. 2014. Biomining: metal recovery from ores with microorganisms. Advances in Biochemical Engineering and Biotechnology, vol. 141. pp. 1-47. [ Links ]
Sherlock, W.K. 2010. Issues affecting heap biooxidation of low-grade refractory gold ore: formation of secondary sulfates, ore lithology, alteration and sulfide mineralogy at Gold Quarry, Carlin, Nevada. MSc thesis. University of Nevada, Reno, NV. [ Links ]
Singer, P.C. and Stumm, W. 1970. Acidic mine drainage: the rate-determining step. Science, vol. 167, no. 3921. pp. 1121-1123. [ Links ]
Stott, M.B., Watling, H.R., Franzmann, P.D., and Sutton, D. 2000. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Minerals Engineering, vol. 13, no. 10, pp. 1117-1127. [ Links ]
Watling, H.R., Shiers, D.W., and Collinson, D.M. 2015. Extremophiles in mineral sulphide heaps: some bacterial responses to variable temperature, acidity and solution composition. Microorganisms, vol. 3, no. 3. pp. 364-390. [ Links ]
Whitlock, J.L. 1997. Biooxidation of refractory gold ores (the Geobiotics process). Biomining. Springer, Berlin/Heidelberg. pp. 117-127. [ Links ]
Xie, X., Yuan, X., Liu, N., Chen, X., Abdelgadir, A., and Liu, J. 2013. Bioleaching of arsenic-rich gold concentrates by bacterial flora before and after mutation. Biomed Research International, vol. 2013. http://dx.doi.org/10.1155/2013/969135 [ Links ]
Zeng, S., Li, J., Schumann, R., and Smart, R. 2013. Effect of pH and dissolved silicate on the formation of surface passivation layers for reducing pyrite oxidation. Computational Water, Energy, and Environmental Engineering, vol. 2, no. 2. pp. 50-55 [ Links ]
Zhou, H.B., Zhang, R.B., Hu, P.L., Zeng, W.M., Xie, Y.J., Wu, C.B., and Qiu, G.Z. 2008. isolation and characterization of Ferroplasma thermophilum sp. nov., a novel extremely acidophilic, moderately thermophilic archaeon and its role in bioleaching of chalcopyrite. Journal of applied microbiology, vol. 105, no. 2. pp. 591-601. [ Links ] ♦
Paper received Apr. 2016
Revised paper received Jan. 2017














