Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.117 n.4 Johannesburg Apr. 2017
http://dx.doi.org/10.17159/2411-9717/2017/v117n4a7
GENERAL PAPERS
Recovery of iron from red mud by high-temperature reduction of carbon-bearing briquettes
T. Chun; D. Li; Z. Di; H. Long; L. Tang; F. Li; Y. Li
School of Metallurgical Engineering, Anhui University of Technology, Maanshan, China
SYNOPSIS
Red mud is the waste generated during the production of alumina from bauxite. The high-temperature reduction of red mud in the absence and presence of sodium borate was carried out to facilitate the subsequent recovery of iron by wet magnetic separation. Sodium borate was found to enhance the recovery of iron, as well as increasing the particle size of metallic iron significantly. High-temperature reduction in the presence of 4% sodium borate increased the iron grade of the metallic powder to 90.05%, compared to 80.24% without sodium borate under the optimum conditions of roasting at 1300°C for 30 min. Experimental evidence showed that sodium borate promotes the reduction of iron oxides and the growth of metallic iron grains, which leads to improved separation between iron and gangue during the subsequent magnetic separation.
Keywords: red mud, sodium borate, high-temperature reduction, metallic iron powder.
Introduction
Red mud is a solid waste residue formed after the caustic digestion of bauxite during the production of alumina. The production of 1 t of metallic aluminum generates about 0.8-1.5 t of red mud (Zhou, Fan, and Li, 2008; Mishra, Syslry, and Kirkpatrick, 2001). In China, about 30 Mt of red mud is produced every year, and about 200 Mt of red mud is stacked (Zhu et al., 2012). Red mud is a highly alkaline solid waste with pH 10-12.5, containing iron, aluminum, silicon, and other nonferrous metals (Gupta and Sharma, 2002; Chun et al., 2014b; Soner, Altundogan, and Fikret, 2002). Red mud has an adverse impact on the environment, and its proper disposal presents a huge challenge for alumina producers.
To solve the disposal problems, many technologies have been proposed for the utilization of red mud, such as the recovery of valuable metals including iron, aluminum, titanium, and other trace metals, ceramic production, and catalysts for waste gas and liquid cleaning (Pontikes, Nikolopoulos, and Angelopoulos, 2007; Chun et al., 2014a; Zhang, Deng, and Xu, 2005; Yang et al., 2006; Amritphale, Avneesh, and Navin, 2007). In recent years, direct reduction and magnetic separation technology has been developed to treat iron-bearing wastes such as copper and chromium slags, pyrite cinder, and red mud (Long et al., 2015, 2016; Chun, Long, and Li et al., 2015; Guo et al., 2016). During reduction roasting, iron oxides are reduced to metallic iron, which could be upgraded by magnetic separation. Previous research reported that the sodium borate is an effective additive to improve the reduction of iron oxides and the growth of the metallic iron grains (Long et al., 2016). Therefore, sodium borate was employed as an additive in this investigation.
Experimental
Raw materials
The red mud used in this study was collected from Shandong Aluminum Corporation, China. The chemical composition was determined by X-ray fluorescence (XRF) (Table I). The major constituents are Fe (42.45% total), SiO2 (6.89%), and Al2O3 (10.34%). The phase composition of iron minerals was tested by X-ray diffraction (XRD), and most of the iron is in the form of limonite and martite, with some magnetite, iron silicate, and iron carbonate (Table II). The size distribution is 80% passing 0.10 mm using the sieve.
Analytical grade sodium borate was employed as an additive during the experiment. The coal used as the reductant had a fixed carbon content of 78.99% (air-dry basis), volatile matter 7.40% (dry ash-free basis), and ash 10.95% (air-dry basis) including 5.69% SiO2, 1.41% Fe2O3, 3.01% Al2O3, 0.64% CaO, and 0.20% MgO. The size distribution was 100% passing 1 mm and 70% passing 0.074 mm.
Experimental method
The experimental process comprised briquetting, high-temperature reduction of briquettes, and magnetic separation, as shown in Figure 1.
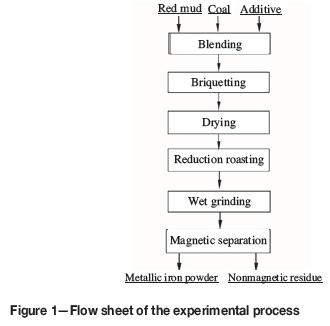
Red mud, sodium borate, and coal fines were mixed in a mixer (CH-10) with 8% water (dry basis) for 1 hour. Cylindrical briquettes 10 mm in diameter and 10 mm in height were produced by pressing 2.5 g of the mixture in a cylindrical mould for 1 minute using a pressure of 8 MPa applied by a vertically loading piston. The briquettes were oven-dried at 105°C for 4 hours.
A graphite crucible 100 mm in diameter and 100 mm in height, containing 50 g dried briquettes, was placed in the furnace. After the designated reaction time, nitrogen was introduced at a flow rate of 1 L/min. The reduced briquettes were ground to 90% passing 0.074 mm in a cone ball mill (model XM0240x90 from Wuhan Rock Crush & Grind Equipment Manufacture Co. Ltd). Magnetic separation was performed on the powdered briquettes using a Davis tube (model XCGS-73) at a magnetic intensity of 0.08 T (Long et al., 2016).
The structures and elemental distribution of reduced briquettes were analysed by scanning electron microscopy (SEM). The chemical composition of the red mud was analysed by X-ray fluorescence (XRF), and the total iron content and metallic iron content in the iron concentrate (metallic iron powder) were determined by chemical method according to the Chinese standard (GB/T6730.6-2016). The degree of metallization was calculated using Equation [1]:

where λ is the degree of metallization (%), ßFeis the total iron content of the metallic iron powder (wt%), and ßMFeis the metallic iron content (wt%).
The iron recovery was calculated as:

where γ is the yield of metallic iron powder (wt%), ßFeis the total iron content of the powder (wt%), and aFeis the total iron content of the reduced briquettes (wt%). Each experiment was carried out in duplicate under the same conditions, and the error bars were less than 1%. The average of the two tests was used as the final result.
Results and discussion
Sodium borate addition
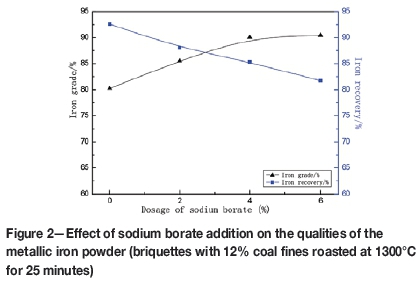
Figure 2 shows the effect of sodium borate addition on the quality of the metallic iron powder. The iron grade increased significantly from 80.04% to 90.43% as the sodium borate dosage was increased from zero to 6%; however, the iron recovery decreased from 92.58% to 81.70%. This can be attributed to the presence of Na2O during the reduction roasting, which could promote the transformation of Fe3+ to Fe0, improving the reduction of iron oxides (Chun et al., 2014). On the other hand, sodium borate also creates more lattice defects in metallic iron, thus promoting recrystal-lization of the metallic iron (Long et al., 2016), which leads to the growth and interconnection of metallic iron grains, resulting in the high iron grade. However, the liquid phase content increased in the presence of the low-melting-point additive (sodium borate). Some metallic iron particles coated by the liquid phase could not be recovered by magnetic separation, thus lowering the iron recovery.
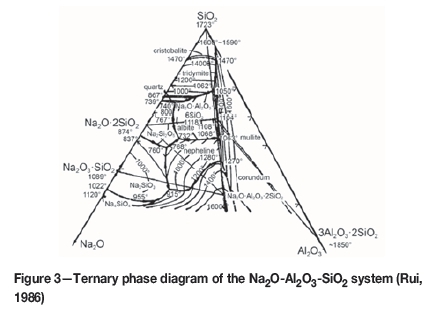
As shown in Table I, the main gangue minerals in the red mud are Al2O3 and SiO2. Figure 3 shows the ternary phase diagram of the Na2O-Al2O3-SiO2 system. The melting point decreased when some Na2O was added in the briquettes. During the reduction roasting, the Na2O decomposed from the sodium borate promotes the formation of low-melting-point minerals, such as Na2O.2SiO2 and Na2O.Al2O3. The liquid phase promotes the transfer and migration of metallic iron and the formation of large particles.
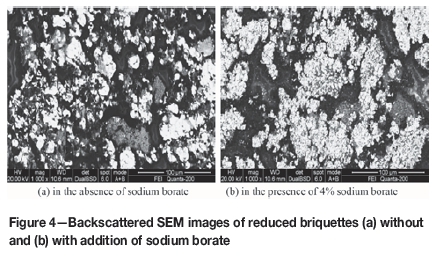
In order to illustrate the mechanisms by which sodium borate improved the reduction of iron in red mud, SEM images of reduced briquettes without and with additions of sodium borate were investigated. Without additions of sodium borate the metallic iron particles in the reduced briquettes were dispersed, with an average particle size of less than 50 μΐη (Figure 4a). With the addition of 4% sodium borate under the same roasting conditions, the metallic iron particles agglomerated and formed larger particles of more than 50 μηι (Figure 4b). Sodium borate addition also forms a lower melting oxide phase, which promotes the growth of metallic iron particles. Larger particles are more easily liberated during milling, and thus are separated more effectively during magnetic separation (Chun, Long, and Li, 2015).
Reduction temperature
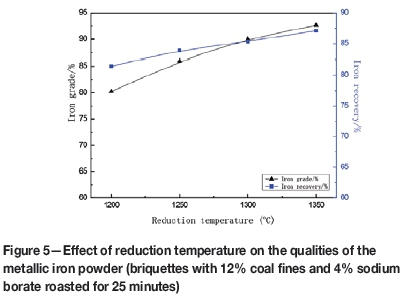
Figure 5 illustrates the effect of reduction temperature on the qualities of the metallic iron powder. Since reduction roasting is an endothermic reaction, increasing the temperature is an effective way to improve the reduction of iron oxides. The higher the reduction temperature, the higher the iron grade and iron recovery to the concentrate. When the reduction temperature was increased from 1200°C to 1350°C, the concentrate grade increased from 80.23% to 92.64% Fe, and the iron recovery increased from 81.45% to 87.11%. In consideration of the increased energy consumption at higher roasting temperatures, a temperature of 1300°C is recommended.
Reduction time
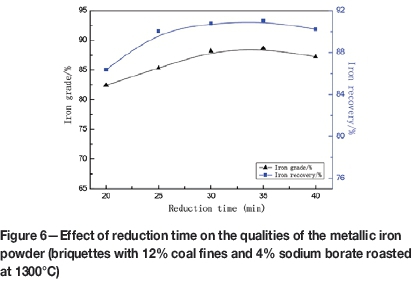
The effect of reduction time on the qualities of the metallic iron powder is presented in Figure 6. When reduction time was extended from 20 to 30 minutes, the iron grade increased from 86.37% to 90.78%, and then levelled off. The iron recovery followed a similar trend. The optimal reduction time is 30 minutes at 1300°C.
Addition ofcoal fines
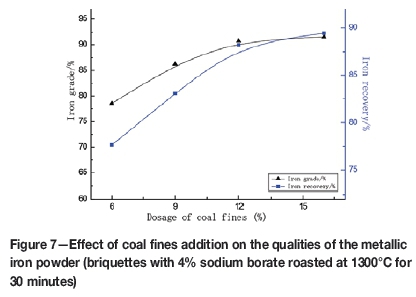
Since the total iron content of the red mud is 42.45% and the fixed carbon content of the coal fines is 78.99%, then according to the reaction 2Fe2O3+3C→4Fe+3CO2, the coal fines addition required is 7.86% in the mixture of red mud and coal fines. Figure 7 presents the effect of coal fines addition on the qualities of the metallic iron powder. The coal fines content of the briquettes is also an important factor during high-temperature reduction. The greater the coal fines content, the more reducing the atmosphere, which promotes the reduction of iron oxides. With coal fines additions of less than 7.86%, the iron oxides could not be reduced fully. However, increasing the addition of coal fines increases the cost. Therefore a coal fines addition of 12% is suggested.
Analysis of final product
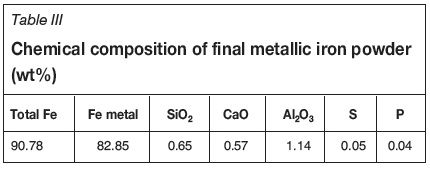
The final metallic iron powder, assaying 90.78% Fetotal (representing a metallization degree of 91.26%) at 88.20% iron recovery, was obtained with additions of 4% sodium borate and 12% coal fines, and reduction at 1300°C for 30 minutes. The chemical composition of final metallic iron powder obtained is shown in Table III.
For the material requirement of the electric arc furnace (EAF), the iron grade and the metallization degree must be higher than 88% and 90%, respectively, and the S and P each less than 0.06%, respectively (Fang, 1994). The iron grade of iron concentrate was higher than 90% and the contents of sulphur and phosphorus were low. The final product, after briquetting, can be used as the feed for steelmaking by EAF, replacing scrap steel.
Conclusions
The iron in the red mud is mainly contained in limonite and martite. High-temperature reduction in the presence of sodium borate followed by magnetic separation is an effective way to recover the iron. During the reduction roasting, Na2O decomposed from sodium borate promotes the reduction of Fe3+ to Fe0 and the growth of metallic iron grains.
A final metallic iron powder, assaying 90.78% Fetotal and representing a metallization degree of 91.26% at 88.20% iron recovery, was obtained under the optimum conditions. The final product can be briquetted for use as feed to steelmaking in an electric arc furnace to replace scrap.
Recommendations
The technical feasibility of producing high-grade metallic iron powder from red mud has been demonstrated. In further work, a pilot-scale experiment will be conducted using a rotary hearth furnace, which is used commercially to treat zinc-bearing dust in Chinese iron and steel plants. As shown in Table III, the Al2O3 content of the metallic iron powder was 1.14% in, which indicates that most of the aluminum reports to the nonmagnetic tailings, and the recovery of alumina from the tailings will be investigated in further research.
It is ineffective to treat refractory secondary iron-bearing wastes such as red mud using traditional technologies, such as gravity separation or flotation. Compared with the traditional technologies, the operating cost of sodium salt reduction roasting is high, but this technology opens the way for the utilization of these wastes.
Compared with iron ore, the red mud is very cheap. The raw material cost could be decreased by replacing some of the coal fines with blast furnace dust, which has a high carbon content. Meanwhile, selection of an efficient reactor is also important in keeping costs down. For example, the rotary hearth furnace is superior to the rotary kiln and tunnel kiln. In industrial application, a good seal is an effective way to improve the reducing atmosphere, which will further decrease the fuel cost. Although the treatment cost is high, the utilization of red mud confers social and environmental benefits.
Acknowledgement
The authors are grateful to the projects of National Natural Science Fund Anhui Province (1608085QE94), the Student Research Training Program (201510360117), the National Natural Science Fund China (51504003), and the Key Project of Natural Science Research of Anhui Universities (KJ2015A028) for sponsoring the research work.
References
Amritphale, S.S., Avneesh, A., and Navin, C. 2007. A novel process for making radiopaque materials using bauxite-red mud. Journal of the European Ceramic S ociety, vol. 27. pp. 1945-1951. [ Links ]
Chun, T.J., Zhu, D.Q., Pan J., and He, Z. 2014a. Recovery of alumina from magnetic separation tailings of red mud by Na2CO3 solution leaching. Metallurgical and Materials Transactions B, vol. 45, no. 3. pp. 827-832. [ Links ]
Chun, T.J., Zhu, D.Q., Pan J., and He, Z. 2014b. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Canadian Metallurgical Quarterly, vol. 53, no. 2. pp. 183-189. [ Links ]
Chun, T.J., Long, H.M., and Li, J.X. 2015. Alumina-iron separation of high alumina iron ore by carbothermic reduction and magnetic separation. Separation Science and Technology, vol. 50, no. 5. pp. 760-766. [ Links ]
Fang, J. 1994. Non-coking Ironmaking. Metallurgical Industry Press, Beijing. p. 185. [ Links ]
Guo, Z., Zhu, D., Pan, J., Wu, T., and Zhang, F. 2016. Improving beneficiation of copper and iron from copper slag by modifying the molten copper slag. Metals, vol. 6. pp. 86-102. [ Links ]
Gupta, V.K. and Sharma, S. 2002.Removal of cadmium and zinc from aqueous solutions using red mud. Journal of Environmental Science and Technology, vol. 16. pp. 3612-3617. [ Links ]
Long, H.M., Chun, T.J., Di, Z.X., Wang, P., Meng, Q.M., and Li, J.X. 2016. Preparation of metallic iron powder from pyrite cinder by carbothermic reduction and magnetic separation. Metals, vol. 6, no. 4. pp. 88-96. [ Links ]
Long, H.M., Meng, Q.M., Wang, P., Chun, T.J., and Yao, V.L. 2015. Preparation of chromium-iron metal powder from chromium slag by reduction roasting and magnetic separation. Journal of Iron and Steel Research International, vol. 22, no. 9. pp. 77-1776. [ Links ]
Mishra, B., Syslry, A., and Kirkpatrick, D. 2001. Recovery and utilization of iron from red-mud. Light Metals Proceeding of Sessions, TMS Annual Meeting. pp. 149-156. [ Links ]
Pontikes, Y., Nikolopoulos, P., and Angelopoulos, G.N. 2007. Thermal behavior of clay mixtures with bauxite residue for the production of heavy-clay ceramics. Journal of the European Ceramic Society, vol. 27. pp. 1645-1649. [ Links ]
Rui, L. 1986. Physical Chemistry of Inorganic Materials. Chinese Construction Industry Press, Beijing. Vol. 194. [ Links ]
Soner, H., Altundogan, S.A., and Fikret, T. 2002. Arsenic adsorption from aqueous solution by activated red mud. Waste Management, vol. 22. pp. 357-363. [ Links ]
Yang, J.K., Fan, C., Hou, J., Xiao, B., and Liu, W. 2006. Engineering application of basic level materials of red mud high level pavement. China Municipal Engineering, vol. 1 23. pp. 7-9 (in Chinese). [ Links ]
Yang, J.K., Hou, J., and Qi, B. 2006. Pilot scale production and industrialization of the no-fired bricks from red mud in aluminum industry. Environmental Engineering, vol. 24, no. 4. pp. 52-55. [ Links ]
Zhang, J.J., Deng, Z.G., and Xu, T.H. 2005. Experimental study on acid leach of red mud. Light Metals, vol. 2. pp.13-15 (in Chinese). [ Links ]
Zhou, Q.S., Fan, K.S., and Li, X.B. 2008. Alumina recovery from red mud with high iron by sintering process. Journal of Central South University (Natural Sciences), vol. 1. pp. 92-94 (in Chinese). [ Links ]
Zhu, D.Q., Chun, T.J., Pan, J., and He, Z. 2012. Recovery of iron from high-iron red mud by reduction roasting with adding sodium salt. Journal of Iron and Steel Research International, vol. 19, no. 8. pp. 1-5. [ Links ]
Paper received Jun. 2016
Revised paper received Nov. 2016














