Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.12 Johannesburg Dec. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n12a11
GENERAL PAPERS
Thermodynamic analysis and experimental studies of magnesium extraction from szaibelyite-suanite ore by aluminium
J.P. PengI, II ; X.L. WuI, III; Y.Z. DiI, II; N.X. FengI, II ; S.G. ZhouI
ISchool of Material & Metallurgy, Northeastern University Shenyang, Liaoning, China
IIKey Laboratory for Ecological Metallurgy of Multimetallic Mineral (Ministry of Education), Northeastern University, Shenyang, Liaoning, China
IIIXi'an University of Architecture and Technology, Xi'an, Shanxi, China
SYNOPSIS
Szaibelyite-suanite type ore is rich in magnesium oxide and boron oxide. We propose a new method to extract both metallic magnesium and valuable residues rich in boron by reduction using metallic aluminium.
Thermodynamic analysis of the reactions between aluminum and MgO, MgjB2O5, and Μ&Β206 was carried out. The effects of CaF2 addition, temperature, mass of Al, and pellet formation pressure on the extraction of magnesium were also investigated experimentally.
The results indicate that the magnesium oxide phase can be displaced from 2MgO-B2O3 and 3MgOB2O3 by CaO in the reduction system. The reduction ratio of magnesium oxide was 38% without calcium fluoride addition, increasing to 94% with 5.1% calcium fluoride. The reduction ratio increased with increasing temperature and mass of Al. To obtain a higher reduction ratio, the reduction pellet should be formed under a reasonable pressure.
Keywords: szaibelyite-suanite, magnesium, aluminum, thermodynamic analysis.
Introduction
Boron and its compounds are widely used in textiles, borosilicate glass, metallurgy, fertilizers, and nuclear shielding. Boron resources are mainly found in Turkey, the USA, Russia, and China. In Liaoning, China, there are abundant szaibelyite-suanite type ores that are rich in magnesium oxide and boron oxide. Szaibelyite and suanite ores are used to produce borax by a carbon dioxidesoda process. Annually, 2 Mt of waste materials containing 3-20% B2O3 and 2-25% MgO are produced during the process. It is necessary to make use of these waste materials containing high concentrations of boron oxide and magnesium oxide to avoid environmental problems as well as for economic reasons (Özdemir and Klpgak, 2007).
Many studies have been performed on boron recovery from boron-containing waste materials (Özdemir and K1pçak, 2007; Wang 2012; Özdemir and K1pçak, 2003). Some studies have focused on the preparation of magnesium sulphate, refractory materials, and fire retardants. Metallic magnesium can be extracted from borax sludge by vacuum thermal reduction (Wu et al., 2009). Boron oxide remains in the reduction residue, and thus the residue can be used to produce nonalkali glass fibre.
Szaibelyite-suanite type ores can be used to produce metallic magnesium using calcium carbide (Peng et al., 2011), metallic silicon (Wu et al., 2011), and metallic aluminum as reducing agents. Furthermore, the reduction residue also contains the main components of non-alkali glass fibre.
In this study, thermodynamic and experimental investigations of magnesium extraction by metallic aluminum were carried out.
Raw materials
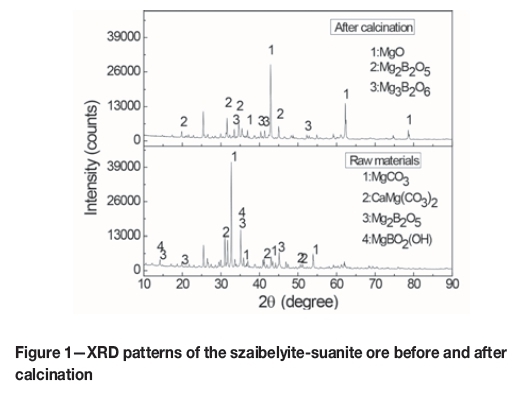
The szaibelyite-suanite ore was supplied by the Kuandian boron-magnesium plant in Liaoning Province, China. Table I lists the chemical composition of the ore. Figure 1 shows a comparison of the X-ray diffraction (XRD) patterns of the ore before and after calcination. The XRD patterns show that the raw ore contains mainly MgCO3, Mg2B2O5, and MgBO2(OH). After calcination, the product consists of MgO, 2MgOaB2O3, and 3MgO-B2O3. Thermodynamic considerations From a comparison of the XRD patterns (Figure 1), it is clear that MgO and 2MgO-B2O3 were generated through several important decomposition reactions during the calcination process:


The CaCO3 generated from Equation [2] decomposes at high temperatures:

The existence of 3MgO-B2O3 proves that a reaction between MgO and 2MgO-B2O3 occurred:

According to Hauck and Muller (1979), the formation of 3MgO-B2O3 occurs easily at high temperatures, and the standard Gibbs free energy ∆G°(in kJ mol-1) for Equation [5] can be calculated by

Based on thermochemical data (Liang and Che, 1993), thermodynamic analysis of the reduction of magnesium oxide by aluminum proceeds as follows:

For 2MgO B2O3 and 3MgO B2O3, we first consider the following possible reactions:

The relationships between equilibrium magnesium partial pressure and temperature calculated from Equations [6], [7], [8], [9], and [10] are plotted in Figure 2. From Figure 2, it is evident that reaction [7] easily takes place because its critical reaction temperature is lower than that of reaction [6] under the same magnesium partial pressure. At temperatures below 2000 K, the Gibbs free energy change of reaction [8] is positive.

The boiling point of magnesium (1363 K) is lower than that of aluminum (2723 K). When the magnesium produced is removed as vapour, reactions [6] and [7] can be accelerated. In addition, reaction [8] can also occur when the equilibrium magnesium partial pressure is decreased, as shown in Figure 2. Hong et al. (1999) and Yang et al. (2006) proposed that the reduction of magnesium oxide by aluminum should take place in two stages: reaction [7] to produce magnesium vapour and MgO·Al2O3 spinel and reduction (reaction [8]) of the spinel by aluminum.
However, Figure 2 indicates that the equilibrium partial pressures of magnesium for reactions [9] and [10] are less than 10-3 atm. below 1500 K. Thus, it is difficult to directly extract magnesium from 2MgO·B2O3 and 3MgO·B2O3.
We propose to add CaCO3 into the reduction system, which is converted to CaO at high temperatures, because some reactions between CaO and B2O3 can easily take place (Turkdogan, 1980):

Therefore, in the reduction system with CaO, we consider the following reactions:

Figure 3 shows the relationships between equilibrium magnesium partial pressure and temperature calculated from Equations [14]-[19].

It is clear from Figure 3 that reaction [16] is most likely to occur because it has the highest equilibrium partial pressure of magnesium vapour. In addition, the figure shows that the equilibrium partial pressures of magnesium for reactions [14]-[19] are higher than 0.01 atm. at 1500 K; i.e., these reduction reactions with CaO could easily occur under vacuum conditions.
Experimental procedure and apparatus
Figure 4 shows a flow diagram of the experimental procedure. First, szaibelyite-suanite powder of less than 125 μm in size and CaCO3 were mixed prior to compaction into briquettes. The briquettes were calcined at 1273 K for 30 minutes in a resistance furnace to eliminate H2O and CO2.

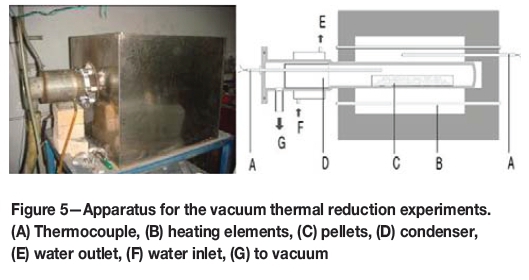
The calcined products were then ground into powder of particle size less than 120 μm. A homogeneous mixture of the calcined product powder and aluminum powder was compacted into pellets 27 mm in diameter and about 10 mm in thickness. Pellet samples were then fed into a retort fitted with a condenser to collect metallic magnesium. The retort was heated to a given experimental temperature and held at this temperature for a given period. The pressure in the retort was controlled by a vacuum system. Figure 5 shows a photograph of the apparatus and a schematic diagram of the retort.
In the present study, experiments were performed at various operation conditions to study the effects of CaF2 addition, temperature, and pellet formation pressure on the reduction ratio of magnesium oxide. In general, the pellet mass was 130-150 g.
Results and discussion
Table II lists the experimental conditions and results of the reduction experiments.
In Table II, the reduction ratio of magnesium oxide is used to estimate the level of the reduction process. It is defined as the ratio between the mass of magnesium extracted and the initial magnesium mass in the pellet used for reduction:

where m0is the pellet mass before reduction, m1is the mass of the residue after reduction, and m* is the mass of magnesium contained in the pellet.
If only magnesium vapour is released from the pellet, the change in the pellet mass is taken as the mass of magnesium extracted. In fact, the magnesium products contained a low level of calcium. At high temperatures, calcium oxide in the pellet would also react with aluminum and form metallic calcium. Nevertheless, most of the metallic calcium would rapidly displace magnesium and form calcium oxide (Liang and Che, 1993):
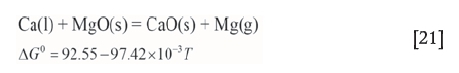
Hence, a very small amount of calcium is released from the pellet. The change in the pellet mass is taken as the mass of magnesium vapour because the mass of calcium is ignored. The error is likely to be less than 2%.
Role of CaC03 addition
An additional experiment was performed to study the behaviour of CaO during the calcination process. The material mixture consisted of 80.00 g ore powder and 151.10 g lime. The briquettes were formed under a pressure of 135 MPa for 2 minutes and were then calcined at 1273 K for 30 minutes.
Figure 6 shows XRD patterns of the mixed material after calcination. The products contained CaO, MgO, and Ca3B2O6. Comparison with the components of the raw ore after calcination (see Figure 1) indicates that the following reactions occurred during the calcination process:


Thus, the magnesium oxide phase is displaced from 2MgO·B2O3 and 3MgO·B2O3 by CaO to form 2CaO·B2O3 and 3CaO·B2O3. Therefore, magnesium can be extracted from szaibelyite-suanite ore by the addition of CaCO3.
Effect of CaF2 addition on the reaction
The Pidgeon process is the main method of magnesium metal production. In the process, calcium fluoride is added to the mixture of calcined dolomite and FeSi to improve the reduction ratio of magnesium oxide. In this study, a small amount of CaF2 was also added into the mixture (see Figure 4). The effect of CaF2 addition on the reduction ratio is shown in Table II.
For experiment C-1 without CaF2, the reduction ratio of magnesium oxide is lower than for the experiments with CaF2 under the same conditions. Figure 7 shows XRD patterns of the reduced residue from C-1. The chemical compositions of the residues from experiments C-1 and C-4 are listed in Table III. The residues contain mainly 3CaO·B2O3, 12CaO·7Al2O3, spinel (MgO·Al2O3), MgO, and Al. The existence of spinel proves that reaction [7] occurred. Calcium aluminate (12CaO·7Al2O3) forms by the reaction between calcium oxide and alumina:



The formation of 12CaO·7Al2O3 favours the reduction of MgO by aluminum, and the following reaction takes place:

However, for experiment C-1, the high content of MgO in the residue verifies that the reduction ratio of magnesium oxide is low. The experimental results in Table II show that the reduction ratio of magnesium oxide increases markedly with increasing amounts of CaF2, reaching 94% in experiment C-4.
Figure 7 also shows XRD patterns of the reduction residue from experiment C-4. Compared with the patterns of the residue obtained from experiment C-1, the XRD peaks of MgAl2O4, MgO, and Al for experiment C-4 are less intense. Thus, reactions [6] and [8] occurred.
Effect of temperature on the reaction
Table II also lists the results for experiments performed at various temperatures. The experimental temperatures were set to 1273-1473 K, which are higher than the melting point of pure aluminum in ambient atmosphere (933 K). Molten aluminum in the briquettes easily penetrates into the magnesium oxide phase. Furthermore, molten aluminum favours mass and heat transfer. Thus, the reaction between aluminum and magnesium oxide easily occurs.
The results show a remarkable improvement in the reduction ratio with increasing temperature. For experiment T-1 carried out at 1273 K, the reduction ratio is very poor. At a temperature of 1373 K (experiment T-3), the reduction ratio of magnesium oxide increases to about 30%. With increasing temperature, both the penetration rate of molten aluminum and the rate of release of magnesium vapour increase, so the reduction of MgO by Al is faster. At a temperature of 1473 K (experiment T-5), the reduction ratio is 95%, which is the highest reduction ratio achieved in the present study. Nevertheless, it must be pointed out that 151 g of calcium carbonate was used in experiment T-5. Under the same operation conditions, experiment C-4 achieved a 94% reduction ratio using only 110 g calcium carbonate (see Table II). Thus, experiment C-4 is considered more economical than experiment T-5.
Effect of the reactant stoichiometry on the reaction
According to Equation [7], the maximum reduction ratio of MgO is 75% because of the formation of MgO·Al2O3. If all of the MgO·Al2O3 reacts with Al to form Mg and Al2O3 (Equation [8]), the maximum reduction ratio of MgO is 100%. The molar ratio of Al: MgO must be higher than 2:3.
According to the chemical composition of the szaibelyite-suanite ore (Table I), 80 g of the ore contains 35.74 g of MgO, therefore the stoichiometric requirement of Al is 16.08 g. Figure 8 shows the results of experiments with various amounts of aluminum. With increasing amounts of aluminum, the contact area of MgO with aluminum particles increases and thus the reduction ratio of magnesium oxide improves.

Effect ofpelletizing pressure on the reaction
The effect of the pelletizing pressure on the reaction is shown in Figure 9. For experiment F-1, the uncompacted material was fed directly into the retort for thermal reduction. The reduction ratio for experiment F-1 is 80%. By increasing the pelletizing pressure to 90 MPa, the contact area between aluminum and MgO increases, and thus the reduction ratio increases.

However, with increasing pelletizing pressure, the porosity of the pellet decreases. Lower porosity hinders the release of magnesium vapour and penetration by unreacted aluminum into the magnesium oxide phase. For this reason, the reaction rate slows and the reduction ratio decreases. Figure 9 shows that a reasonable pelletizing pressure is about 90 MPa.
Conclusions
Thermodynamic analysis of the reduction reactions of magnesium in MgO, Mg2B2O5, and Mg3B2O6 by aluminum was carried out. The effects of CaF2, temperature, mass of Al, and pelletizing pressure on the reduction ratio were also investigated experimentally. The following conclusions can be drawn.
(1) It is difficult to directly extract magnesium from 2MgO·B2O3 and 3MgO·B2O3. With CaO in the system, the magnesium oxide phase can be displaced from 2MgO·B2O3 and 3MgO·B2O3 due to the formation of 2CaO·B2O3 and 3CaO·B2O3. Thus, most of the magnesium in szaibelyite-suanite ores can be extracted under reasonable conditions
(2) The reduction of magnesium oxide by aluminum occurs in two stages: the reaction to produce magnesium vapour and MgO-Al2O3 and the reduction reaction between MgO-Al2O3 and aluminum under vacuum conditions. The formation of 12CaO-7Al2O3 favours the reduction of MgO by aluminum
(3) Calcium fluoride addition markedly improves the reduction ratio of magnesium oxide. The reduction ratio increases with increasing experimental temperature: from 3% at 1273 K to 95% at 1473 K
(4) Increasing the pelletizing pressure up to 90 MPa improves the reduction ratio from 83% (zero pressure) to 94%. Further increases in pelletizing pressure decrease the porosity of the pellets, hindering the release of Mg vapour and thus slowing the rate and extent of the reaction.
Acknowledgements
This work was supported by a grant from the Fundamental Research Funds for the Central Universities (N100302009 and N150204013) and the National Natural Science Foundation of China (51304044).
References
Hauck, D. and Muller, F. 1979. Thermochemie des systems MgO-B2O3. Zeitschrift fur Physikalische Chemie Neue Folge, vol. 118. pp. 79-87. [ Links ]
Hong, L., Okumura, K., and Sano, M. 1999. Nonisothermal gravimetric investigation on kinetics of reduction of magnesia by aluminum. Metallurgical and Materials TransactionsB, vol. 30B. pp.1003-1008. [ Links ]
Liang, Y.J. and Che, Y.C. 1993. Handbook of Inorganic Thermodynamics. Northeastern University Press, Shenyang, China. [ Links ]
Özdemir, M. and K1pçak, i. 2003. Boron recovery from borax sludge, boron industrial waste, by solid-liquid extraction. Industrial and Engineering Chemistry Research, vol. 42. pp. 5256-5260. [ Links ]
Özdemir, M. and K1pçak, i. 2007. Boron recovery from borax sludge using solid-liquid extraction followed by sorption with a boron selective resin in column. Environmental Progress, vol. 26, no. 4. pp. 375-383. [ Links ]
Peng, J.P., Wu, X.L., Feng, N.X., Zhou, S.G., and Wang, Z.H. 2011. Experimental study on vacuum thermal reduction of ascharite mineral with calcium carbide as reductant. Vacuum and Surjace Engineering: Proceeding of the 10th International Conference on Vacuum Metallurgy. Ba, D.C. (ed.). Publishing House of Electronics Industry, Beijing. pp. 8-12. [ Links ]
Turkdogan, E.T. 1980. Physical Chemistry of High Temperature Technology. Academic Press, New York, USA. pp. 7-8. [ Links ]
Wang, W.X. 2012. Technology of boron recovery from boron mud in boric acids production from low grade boron ore. Industrial Minerals & Processing, vol. 41, no. 9. pp. 23-25. [ Links ]
Wu, X.L., Feng, N.X., Peng, J.P., and Wang, Y.W. 2009. Vacuum thermal extract magnesium from boron mud. Magnesium Technology 2009. Nyberg, E.A., Agnew, S.R., Neelameggham, N.R., and Pekguleryuz, M.O. (eds). The Minerals, Metals & Materials Society, Warrendale, PA. pp. 61-63. [ Links ]
Wu, X.L., Feng, N.X., Peng, J.P., Wang, Z.H., and Cheng, E.Q. 2011. Experimental study on vacuum thermal reduction of ascharite mineral with silicon as reductant. Chinese Journal of Process Engineering, vol. 11, no. 4. pp. 294-298. [ Links ]
Yang, J., Kuwabara, M., Sawada, T., and Sano, M. 2006. Kinetics of isothermal reduction of MgO with Al. ISIJ International, vol. 46, no. 8. pp. 1130-1136. [ Links ]
Paper received Feb. 2013
Revised paper received May 2016














