Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.12 Johannesburg Dec. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n12a9
GENERAL PAPERS
Extraction of copper and gold from anode slime of Sarcheshmeh Copper Complex
M.H. DehghanpoorI; M. ZivdarI; M. TorabiII
IUniversity of Sistan and Baluchestan, Zahedan, Iran
IISarcheshmeh Copper Complex,Rafsanjan, Iran
SYNOPSIS
A hydrometallurgical route was developed to extract metals such as gold, silver, copper, and selenium from anode slime from Sarcheshmeh Copper Complex in Iran. For the extraction of copper by leaching, the anode slime was heated and stirred with 4 M sulphuric acid in the presence of oxygen. Optimal conditions for recovery of copper were temperature 80°C, mixing time 5 hours, and oxygen flow rate 2 L/min, resulting in a copper recovery of 94.47%. Silver and selenium were dissolved by leaching anode slime from which the copper had been removed with nitric acid. For gold extraction, anode slime was heated with aqua regia at 90°, and the gold then extracted with 2-ethyl-hexanol at a concentration of 0.5 M and phase ratio of 4. The extract was then stripped with soda solution at pH=10.5 and a phase ratio of 5. Finally, gold was recovered by precipitation with oxalic acid at a ratio of mass to volume 10 g/L. Total gold recovery was 80%.
Keywords: solvent extraction, leaching, copper, gold, anode slime, Sarcheshmeh Copper Complex.
Introduction
During the electrorefining of copper, some metals are accumulated in the byproduct anode slime, which is an important source for recycling and recovery of these metals (Petkova, 1994). In this paper, a hydrometal-lurgical route is described for the recovery of precious metals from copper anode slime of Sarcheshmeh Copper Complex, which is located in southeastern Iran .
Copper anode slime is black or gray powder with a particle size less than 200 mesh. Approximately 2 to 20 kg of anode slime is produced per ton of copper cathode. The slime is characterized by higher amount of Ag, Se, Pb, and Cu compared to other metals and a very low Au content (Khaleghi and Ghader, 2013).The copper anode slimes are composed of 11.69% selenium, 3.93% copper, 5.26% silver, 0.13% gold, and 45% barite, plus lead, tellurium, antimony, sulphur, and silica with small amounts of nickel, iron, zinc, and bismuth. Gold is one of the most important noble metals due to its wide applications in industry and economic importance, so recovery of gold from slime is more appealing than that of other metals. According to the World Gold Council (2013), the demand for gold has increased during the last decade. This increase is due not only to the increasing demands of the jewellery market, but also to the increasing demand for gold in industrial as well as medical applications (Ranjbar et al., 2014).
Different methods to extract valuable metals like gold exist, but hydrometallurgical methods are more affordable. Hydrometallurgy consists of leaching - transferring desirable components into solution using acids or halides as leaching agents, purification of the leach solution to remove impurities by solvent extraction, adsorption, or ion exchange, then recovery of base and precious metals from the solution by an electrorefining process, chemical reduction, or crystallization (Ivšić et al., 2009).
Several methods, such as resin adsorption (Donia et al., 2007; Nguyen et al., 2010) or activated carbon have been investigated for recovery of gold from slimes, waste, and other sources. Cyanidation is currently the conventional gold recovery method, but researchers are looking for alternative methods that do not employ cyanide (Ranjbar et al., 2014).
Rice et al. (1988) studied the leaching of copper, silver, and gold from anode slime and non-representational compounds CuSe, Ag2Se, and CuAgSe with nitric acid. Dutrizac and Chen (1990) also studied slime leached with NaCl addition, and the main difference in the leach residue was found to be that silver occurs mainly as silver chloride instead of sulphate, although silver sulphate is also insoluble in sulphuric acid.
Luo et al. (1997) reported a method for treating anode slime in several operations, including removal of copper and nickel by leaching with dilute sulphuric acid, leaching of selenium, silver, and tellurium with dilute HNO3 (2-8 M), recovery and recycling of HNO3, selective solvent extraction of silver, and finally separation and recovery of selenium and tellurium from the Ag-free solution by reduction with SO2 under different conditions (acidities).
Hait et al. (2002) described the role of MnO2 and NaCl in sulphuric acid leaching of copper anode slime. They found that at atmospheric pressure, leaching recovery of Cu with sulphuric acid is low and addition of MnO2 is beneficial for leaching copper, selenium, and tellurium. A combination of MnO2 and NaCl was also investigated, which gave even higher recoveries of these metals and gold removal but was not successful for removing Ag.
Yavuz and Ziyadanogullari (2000) studied the recovery of gold and silver from copper anode slime. The slime contained Cu, Te, and Se in addition to Ag and Au. Pressure leaching with sulphuric acid in an autoclave was used to remove Cu. Selenium and tellurium were extracted by Na2SO3 or Na2S solutions and the remained selenium was removed by roasting. They found that if the roasted samples are leached first with concentrated sulphuric acid in an autoclave at 350°C under 800 kPa oxygen pressure, and then with 0.1 M sulphuric acid followed by 3 M ammonia solution, most of the silver can be dissolved. Gold was recovered by thiourea leaching (Khaleghi and Ghader, 2013).
Paatero and Haapalainen (2013) described a method for extracting gold selectively from acidic chloride-based aqueous solution or solid-containing slurry by solvent extraction to produce pure gold. A di-ester of 2,2,4-trialkyl-1,3-pentanediol was used as the organic extraction reagent. The gold-bearing organic solution was scrubbed with an acidic aqueous solution, after which gold was stripped into water and then reduced to form pure gold.
Pan et al., (2013) reported a new method specifically for silver separating residues of the copper anode slime. Sodium thiosulphate was subjected to mixing and ball milling, and the ball-milled material and a solvent were treated in a hydrothermal reaction still. The product solution was filtered and thiourea dioxide reductant was added to produce coarse gold and silver powders .
Ranjbar et al. (2014) studied the recovery of gold from copper anode slime by means of a novel process utilizing magnetite nanoparticles (MNPs) that were first synthesized by a co-precipitation method. All the gold content of copper anode slime was dissolved in thiourea solution and a positively charged complex of gold and thiourea was obtained as a result. The gold complex adsorbed on the negatively charged MNPs. Ammonia was added to the gold-MNP suspension to precipitate the gold in metallic form.
The purpose of the present research is the efficient recovery of copper and gold from anode slime by solvent extraction and leaching. This process has to be carried out at the optimum operating conditions, and also economically, for possible scale-up.
Materials and methods
The copper anode slime of Sarcheshmeh Copper Complex was used in this study, and the reagents (sulphuric acid 88%, nitric acid 55%, hydrochloric acid 28%, oxalic acid 99.7%, soda 98%, 2-ethyl-hexanol 97%, and benzene) were supplied by Iranian companies. X-ray diffraction (XRD) and X-ray fluorescence (XRF) spectroscopy were used for chemical and mineralogical analysis of the copper anode slime. The XRD and XRF equipment models were Phillips and ARL 8410, respectively.
Leaching of gold in the anode slimes is affected by some elements such as silver, copper, iron, and selenium. The anode slime was first washed with 4 M sulphuric acid to remove all the copper. Sulphuric acid was then added to anode slime in a 1:10 (solid to liquid) phase ratio and the mixture heated on a hotplate in the presence of oxygen while being stirred with a magnetic stirrer. In this stage the effective parameters were temperature, mixing time, and oxygen flow rate.
The precipitate from the previous step was washed with water. Then 4 M nitric acid was added with phase ratio 1:10 (solid to liquid). The mixture was stirred with a magnetic stirrer at 450 r/min and heated on a hotplate directly at 90° for 3 hours. As a result, gold and residual barite were deposited, and silver, selenium, and other light metals were dissolved.
The residual solids that were produced after washing the copper anode slime with nitric acid were leached with aqua regia at a solid to liquid ratio 1:10 and the mixture was stirred and heated at 80 for 1 hour. After filtering the mixture, a solution containing gold was obtained.
For recovering gold from the mixture of gold-containing copper anode slime a two-stage method was used. Gold was selectively extracted from the mixture with an alcohol, and stripped from the alcohol with soda solution. Metallic gold was then precipitated using oxalic acid. All the above processes were carried out at room temperature.
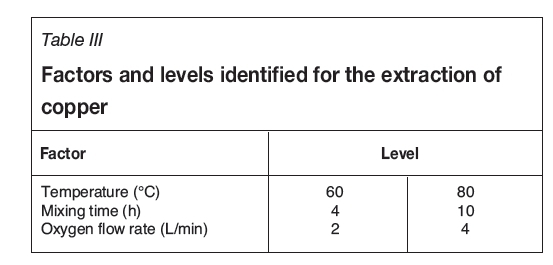
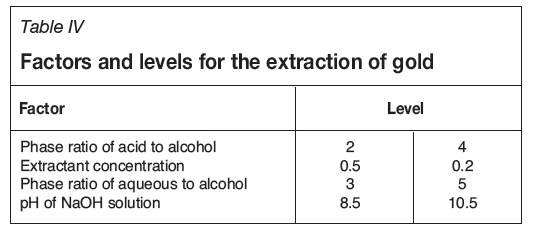
To establish the effect of process parameters on the extraction of gold and copper from anode slime, the Taguchi experimental design method was used. For extraction of copper the three variables are temperature, mixing time, and oxygen flow rate. These variables were studied at two levels. For extraction of gold, the four variables are the phase ratio of alcohol to acid, phase ratio of aqueous phase to alcohol, pH of the NaOH solution, and concentration of alcohol. These variables were studied at three levels. The key variables and levels were selected based on previous research.
Results and discussion
Analysis
The anode slime was collected from the electrolytic cells at Sarcheshmeh Copper Complex. XRF spectroscopy was used for mineralogical characterization of the slime, and the results are shown in Table I. The anode slime analysis by the ICP-MS method is shown in Table II. The anode slime contained approximately 3.5% copper and 0.14% gold.
Design of experiments
The experimental conditions for the extraction of copper and gold from anode slime are shown in Tables III and IV respectively.
Effect of temperature on extraction of copper
The mixing temperature is the parameter that has the greatest effect on copper extraction from anode slime. The results (Table V) show that at the same oxygen flow rate, copper recovery increased significantly with increasing temperature.
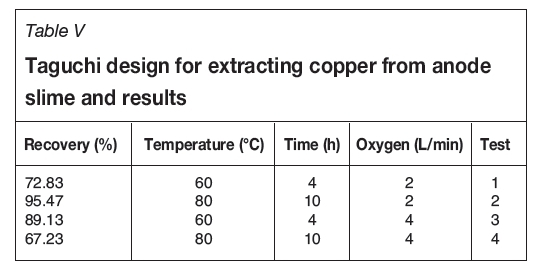
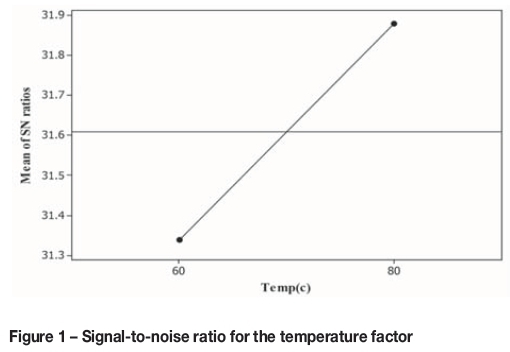
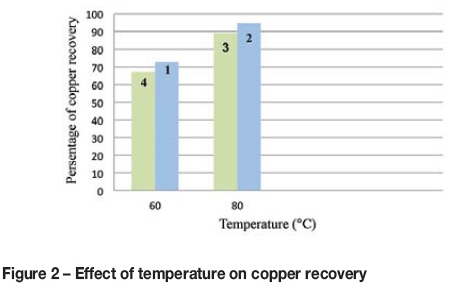
Figure 1 shows the signal-to-noise ratio for the temperature factor. At the higher temperature, where a higher signal-to-noise has been obtained, recovery is higher than the first level. In Figure 2, copper recovery is plotted against temperature for each test. The highest recovery was reached at 80°.
Effect ofmixing time on extraction of copper
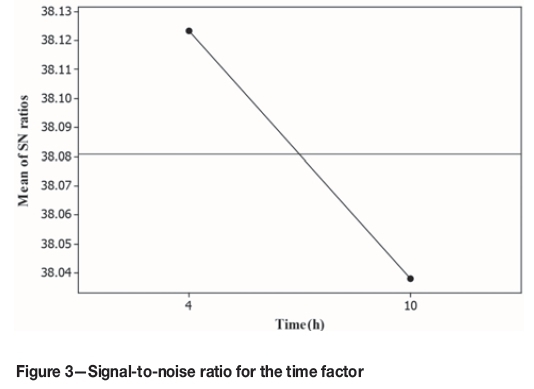
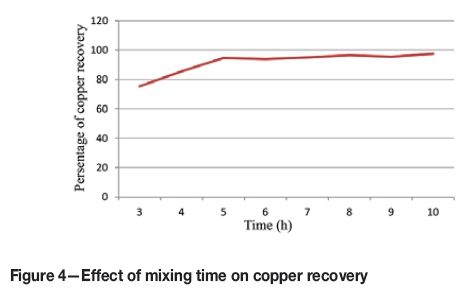
The results in Table VI show that a significant increase in copper recovery was obtained with longer mixing times. Figure 3 shows the values of the signal-to-noise ratio for the time factor. It can be seen that after 5 hours, the recovery remained nearly constant, and thus the best mixing time was considered to be 5 hours.
Effect ofoxygen flow rate on extraction of copper
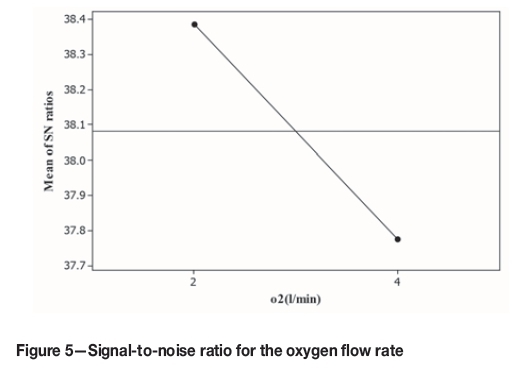
The main purpose of injecting oxygen into the system is oxidation of the copper sulphide within the anode slime to copper oxide. Dissolution of gas in the solution is slow, therefore increasing the gas flow rate did not affect the recovery of copper. Figure 5 shows the values of the signal-to-noise ratio for the oxygen flow rate factor. As shown, the slope is negative and the lower oxygen flow rate was chosen.
Effect ofacid-to-alcohol phase ratio
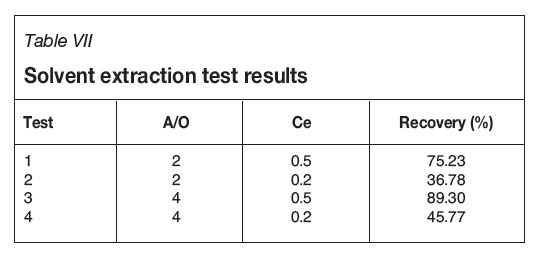
According to the results in Table VII, by increasing the phase ratio of acid to alcohol (A/O) with the same extractant concentration, the gold recovery was increased. As a result, in this research the higher phase ratio was chosen. However, by increasing this ratio, the results were reversed. This is due to the lack of sufficient extractant.
Effect of alcohol concentration
The extractant concentration is the most significant parameter for extraction of gold from anode slime. According to the results shown in Table VII, at the same A/O, gold recovery was increased significantly with increased alcohol concentration. At the best recovery, the phase separation time was 23 seconds.
Effect of aqueous-to-alcohol phase ratio
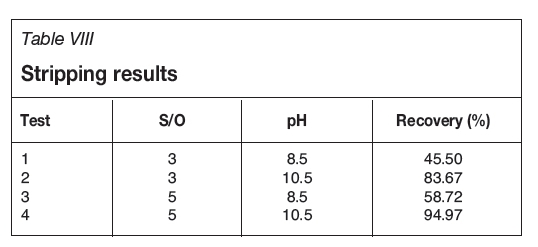
A solution of NaOH was used for gold stripping from the loaded organic (alcohol) phase. The effective parameters were the aqueous-to-alcohol (phase ratio of S/O) and the pH of the NaOH solution, which were studied at two levels. According to the results in Table VIII, by increasing the phase ratio of aqueous to alcohol at the same pH, gold recovery was increased. As a result, the higher phase ratio was chosen. This is due to the complete mixing of the extractant in the presence of soda.
Effect of pH of soda solution
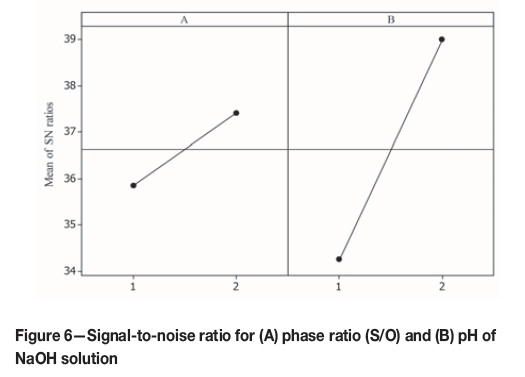
The pH of soda solution is the most significant parameter in the stripping gold from the loaded organic. According to the results shown in Table VIII, at the same ratio of S/O, by increasing the pH, gold recovery was increased significantly. Figure 6 compares the signal-to-noise values for both parameters. For both parameters the signal-to-noise were increased with the change from the first to the second levels, but, the slope of the line for the pH parameter is greater than that for phase ratio (S/O). This shows that adjustment of the pH of the NaOH solution is very important in gold stripping.
Reduction
Oxalic acid was used to precipitate metallic gold from the NaOH solution. The amount of oxalic acid required for the reduction of the dissolved gold was established experimentally. Approximately 10 g of the acid per litre of solution resulted in almost complete reduction of gold. This process was carried out at room temperature. Under the best conditions, a gold recovery of about 81% was obtained.
Conclusions
The maximum copper recovery of 95.47% was obtained at a temperature of 80°C, mixing time of 5 hours, and oxygen flow rate of 2 L/min.
For gold extraction, the best conditions were an acid-to-alcohol phase ratio of 4 and extractant concentration of 0.5 M. For stripping of gold the best condition were an aqueous-to-alcohol phase ratio of 5 and pH 10.5, with reduction to metallic gold using 10 g/L oxalic acid. The gold recovery was 81%.
Acknowledgment
The authors express their gratitude to Sarcheshmeh Copper Complex for providing financial and technical facilities.
References
Dutrizac, J. and Chen, T. 1990. Mineralogical characterization of anode slimes-Part 6, Pressure leached slimes from CCR division of Noranda Minerals. Canadian Metallurgical Quarterly, vol. 29, no.4. http://dx.doi.org/10.1179/cmq.1990.29.4.293 [ Links ]
Donia, A.M., Atia, A.A., and Elwakeel, K.Z. 2007. Recovery of gold(III) and silver(I) on a chemically modified chitosan with magnetic properties. Hydrometallurgy, vol.8, no. 3. pp. 197-206. [ Links ]
Ivsić, D., Kamberovć, Z., Kora , M., and Nikolć, V. 2009. Hydrometallurgical process for extraction of metals from electronic waste - part 1: material characterization and process option selection. Association of Metallurgical Engineers of Serbia. [ Links ]
Habashi, F. 2000. A Text Book of Hydrometallurgy. Abdollahi, M. (trans.). Department of Mining and Metallurgy, University of Shahrood, Iran. [ Links ]
Hait, J., Jana, R.K., Kumar, R.K., and Sanyal, S.K. 2002, Some studies on sulfuric acid leaching of anode slime with additives. Industrial & Engineering Chemistry Research, vol. 41, no. 25. pp. 6593-6599. [ Links ]
Khaleghi, A. and Ghader, S. 2013, Ag recovery from copper anode slime by acid leaching at atmospheric pressure to synthesize silver nanoparticles. Journal of Mining Science and Technology, vol. 24, no.2. DOI: 10.1016/j.ijmst.2014.01.018 [ Links ]
Luo, R., Rice, N.M., Taylor, N., and Gee, R. 1997. A study of the oxidative dissolution of synthetic copper-silver selenide minerals using the intermittent galvanostatic polarization (IGP) technique. Hydrometallurgy, vol. 45. pp. 221-238 [ Links ]
Nguyen, N.V., Jeong, J., Jha, M.K., and Lee, J.C. 2010. Comparative studies on the adsorption of Au(III) from waste rinse water of semiconductor industry using various resins. Hydrometallurgy, vol. 105, no. 1-2. pp. 161-167. [ Links ]
Petkova, E.N. 1994. Hypothesis about the origin of copper electrorefining slime. Hydrometallurgy, vol. 34, no. 3. pp. 343-358. [ Links ]
Paatero, E. and Haapalainen, M. 2013, Method for recovering gold by solvent extraction, US patent EP20110791989. 17 April. 2013. [ Links ]
Pan, D., Zhang, S., Li, B., Tian, J., and Guo, B. 2013. Method for recycling gold and silver from silver separating residues of copper anode slime. Chinese patent CN 102943180 A. 27 February 2013. [ Links ]
Rice, M. 1988. Characterisation and leaching of copper refinery anode slimes. LUMA (Leeds University Mining Association) Magazine. pp. 63-74. [ Links ]
Ranjbar, R., Naderi, M., Amoabediny, G.H., and Omidvar, H. 2014. Gold recovery from copper anode slime by means of magnetite nanoparticles (MNPs). Hydrometallurgy, vol. 143. DOI: 10.1016/j.hydromet.2014.01.007 [ Links ]
World Gold Council. 2013. Gold demand trends. https://www.gold.org/download/file/3262/GDT_04_2013.pdf [ Links ]
Yavuz, O. and Ziyadanogullari, R. 2000. Recovery of gold and silver from copper anode slime. Separation Science and Technology, vol. 35, no. 1. pp. 133-141. [ Links ]
Paper received Apr. 2015.














