Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.12 Johannesburg Dec. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n12a4
GENERAL PAPERS
Optimization of flotation pH for the reverse flotation of an African low-grade BIF haematite ore
A. FoucheeI; N. NaudéI; S. NaikII; K. SchommarzI
IDepartment of Materials Science and Metallurgical Engineering, University of Pretoria, Pretoria, South Africa
IIAnglo American Technical Solutions
SYNOPSIS
This article presents laboratory test work conducted on an African haematite ore to determine the influence of the flotation pulp pH on the final iron grade and recovery. Results show that a combination of an isodecyl ether propylene amine/amino acetate and 1,3-propanediamine, N-(3-(C10-C16-alkyloxy)propyl)-derivatives collector is suitable for separating haematite from quartz. Higher iron grades were obtained at pH levels between neutral and pH 9. This underlines the importance of the surface charge effect of the ore on its flotation characteristics. The results serve as good baseline conditions for further optimization.
Keywords: iron ore, froth flotation, mineral processing, flotation reagents.
Introduction
Beneficiation of iron ore by flotation is widely used in Brazil, India and China. In Africa, however, this practice is not yet commercially developed.
The aim of this test work is to serve as a starting point by establishing suitable pH conditions for the development of a practically implementable reagent suite, which will then be included in a larger beneficiation circuit for producing high-grade iron ore sinter feed.
In the literature, African iron ore types are graded as low-grade (60.0-62.9% Fe; 8.612.5% SiO2 and insolubles), high-grade (66.069.9% Fe; 0.8-4.5% SiO2 and insolubles), and meduim-grade as the remaining portion (Astrup et al., 1998). The low-grade ore used in this work contains less than 60% Fe.
For Brazilian ores consisting of a combination of haematite and quartz, it is common to use corn starch as a depressant and ether amine blends as collectors with additional frothing characteristic (Araujo et al., 2005). Work by Turrer and Peres (2010) shows the possibility of using other depressants successfully as well.
The main mechanism of the reverse flotation of quartz from iron ore is based on the electrostatic theory of flotation. This is due to the strong pH dependence of the zeta potential of the mineral edge planes on silicate particles (Fuerstenau and Pradip. 2005).
An electrical double layer, (inner and outer Helmotz planes), as illustrated in Figure 1, forms on the surface of particles submerged in a solution. The charge in the outer Helmotz plane is depicted by the potential-determining ions on the mineral surface. The ion type that is predominantly concentrated on the surface is determined by the solution pH.
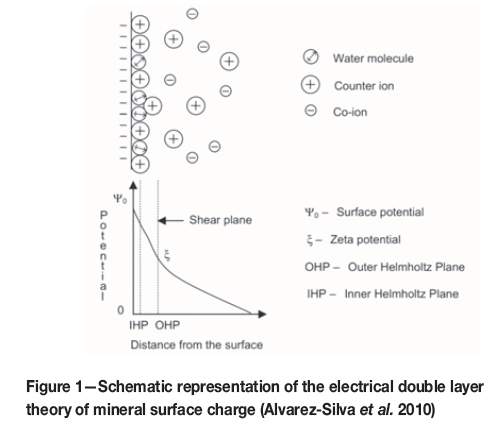
In general, a higher quartz recovery to the froth phase is attained at pH 9, which is attributed mainly to the largest negative surface charge at this pH, (Vieira and Peres, 2007), as indicated in Figure 2. Hydrophobicity of quartz increases as the zeta potential becomes more negative, due to the increased force between the negatively charged quartz surface and the positively charged amine molecule.
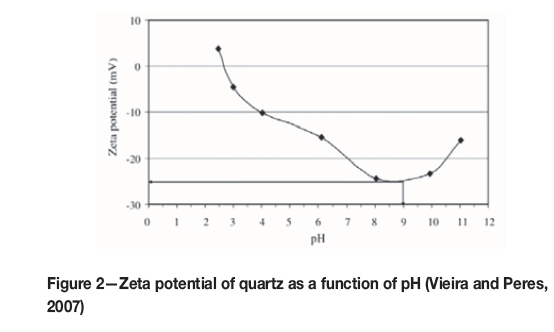
Sirkeci (2000) attributed the increased quantities of silicate minerals reporting to the froth phase to the equilibrium between ionized and molecular amine species at pH 9.3.
Materials and methods
A low-grade haematite ore from Africa was utilized for this flotation test work. Ore characterization consisted of particle size distribution, mineralogical characterization, and compositional characterization. Sample splitting and blending at a representative level were conducted to produce feed samples for laboratory-scale flotation tests.
The flotation reagent suite included 150 g/t combined isodecyl ether propylene amine/amino acetate and 1,3-P-propane-diamine, N-(3-(C10-C16-alkyloxy) propyl)-derivatives collector and a causticized corn starch, with solution concentration of 2.0% by weight. An impeller speed of 800 r/min was maintained for conditioning (at 66% solids by weight) and flotation (at 30% solids by weight). The flotation medium consisted of synthesized process water.
The flotation pulp pH was adjusted by addition of chemical-grade NaOH or H2SO4. Chemical-grade corn starch and dextrin depressants were evaluated, as well as industrial-grade sodium silicate dispersants.
Each feed sample was milled to approximately 80% passing 30 μm in a laboratory-scale mill, with hardened steel rods as grinding media.
Flotation tests were conducted in a Denver-D12 laboratory flotation machine and a 2.6 litre vessel.
Chemical analysis of Fe and SiO2 was carried out using X-ray fluorescence. Mineralogical characterization was performed with by X-ray diffraction.
Results and discussion
The results are presented in terms of a silica rougher flotation circuit. The reverse flotation of iron ore is aimed at increasing the iron content in the silica rougher tails to a saleable product grade.
Ore characterization
Seven samples of iron ore with a banded iron formation (BIF) structure were blended and characterized as a composite. Mineralogical analysis by XRD indicated only the presence of haematite and quartz in the ore. The relative phase amounts, calculated by the Rietveld method (Bergmann and Kleeberg, 1998), amounted to 47.5% haematite and 52.5% quartz.
XRF analysis, however, indicated 47.6% Fe2O3 and 51.4% SiO2. The balance of less than 1.0% (by weight) consisted predominantly of Al2O3 and K2O. This indicated the possibility for minor amounts of additional gangue minerals in the feed.
Grinding curves, shown in Figure 3, were constructed for 10-40 minutes' grinding times. Interpolation of the 20-minute and 30-minute grinding data shows that milling of each sample for approximately 25 minutes would result in the desired feed particle size range of at least 80% passing 30 μm.

Reagent selectivity
The starting point of this test work was to determine a suitable depressant. The iron grade and iron recovery from a laboratory flotation test at each of the individual pH levels with different depressants is shown in Figure 4. Dextrin as a depressant was evaluated only at pH values of 3, 5, and 9.

A baseline test, where no depressant was added to the flotation pulp, showed that the iron-containing mineral, haematite, has the highest natural hydrophobic tendency, (floatability) around pH 7, where a low recovery of Fe2O3 to the silica rougher tails is noted. This tendency decreases at pH levels below and above pH 7 where lower flotation recoveries are obtained, as seen in Figure 4. As also shown in this figure, the normalized iron grade in the silica rougher tails decreases below and above pH 7. This is due to the change in surface charge to where Fe2+ ions predominate on the particle surfaces.
At pH 3, no significant change in either the iron grade or the iron recovery could be established by adding 400 g/t depressant. At pH 5, however, an increase in the iron recovery, from 32.0% to 41.0%, could be attained with starch addition and to 51.0% Fe recovery with Dextrin addition. The decrease in iron grade at pH 5, from normalized 23.1% Fe (where no depressant was used) to between 9.9% and 10.3% Fe (for either dextrin or corn starch) can be attributed to the non-selectivity of the depressants at this pH, thus quartz minerals are also depressed. At pH 9, a slight increase in the iron grade is noticed with the use of starch as a depressant, where a normalized iron grade of 25.9% Fe was obtained. Dextrin at this pH resulted in an iron recovery of 67.0% at a normalized 23.1% Fe grade.
Additional tests at pH 7 showed that the use of starch and no depressant produces similar results. Here the increase to a normalized 30.1% Fe grade (for starch) and 30.4% Fe (for no depressant) is offset by iron recoveries of 23.2% and 21.2%. At pH 11, where Fe3+ ions predominate on the particle surface, a high iron recovery of more than 80% could be attained only at a normalized iron grade of 8.2% Fe (for starch) and 2.4% Fe (for no depressant).
Figure 4 corroborates the theory that the adsorption of the depressant on an oxide mineral surface is driven by the surface charge. In terms of the normalized iron grade in the silica rougher tails, it also shows very little difference between the types of depressant in slightly alkaline conditions.
The addition of a sodium silicate dispersant was tested to improve the selectivity of the reagent suite in conjunction with starch as a depressant. Figure 5 compares a baseline test, where no dispersant used, to the use of 80 g/t and 100 g/t of two dispersant types. The figure also shows the iron recovery obtained using each dispersant type and dosage corresponding to the best normalized iron grade.
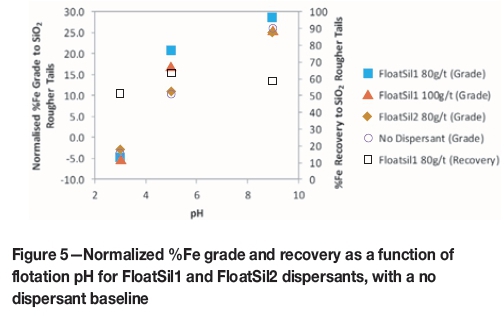
Figure 5 shows that when using 80 g/t FloatSil1 as a dispersant, the normalized iron grade was increased to 28.4% Fe at pH 9 and 20.5% Fe at pH 5. Using this dispersant resulted in an iron recovery of 63.5% at pH 7 and 58.2% at pH 9. The use of FloatSil2 at pH 5 resulted in no improvement in terms of the normalized iron grade, compared to a test where no dispersant was used. Changing the dispersant type to FloatSil2 or increasing the FoatSil1 dispersant dosage to 100 g/t did not result in any significant improvement in the iron grade at pH 9.
Selectivity from pulp basicity
The influence of the pulp pH in the laboratory flotation test is more significant than the influence of the starch depressant dosage over a broad pH range, as shown in Figure 6. The iron grade in the silica rougher tails seems to be influenced only marginally by an increase of depressant dosage from 100 g/t up to 800 g/t (especially at pH 9).

Figure 6 shows that it would be beneficial during reverse flotation to keep the flotation pH range between neutral and slightly alkaline, when the higher normalized iron grade values at pH 7 and pH 9 are considered.
This test work is to be used for further optimization studies, thus it is critical to consider the recovery of iron minerals to the product as well. Turrer and Peres (2010) noted good flotation performance in the region of more than 40% Fe recovery.
Figure 7 shows that although the normalized iron grade in the silica rougher tails could be increased to above 29% Fe at pH 7, the higher iron recovery at pH 9, (more than 35%), irrespective of the depressant dosage, makes pH 9 a more suitable pH level for reverse flotation of this African iron ore. This again confirms the strong dependence of the flotation result on the surface charge of the floated mineral (quartz), as a result of the specific pulp pH environment.

Conclusions
The test work presented in this article forms part of a more complete and in-depth study and shows that for the reverse flotation of iron ore, the flotation pH is more significant than the depressant types and dosage. It also underlines the importance of the surface chemical effects and indicates that flotation in a slightly alkaline pulp environment, where better iron recoveries are attainable, serves as good baseline conditions for further flotation optimization.
Acknowledgements
The authors thank Anglo American for financial support of this project, Anglo American Technical Solutions for the preparation of the ore, and William le Roux, the milling assistant, for flotation work at the University of Pretoria.
References
Alvarez-Silva, M., Uribe-Salas, A., Mirnezami, M., and Finch, J.A. 2010. The point of zero charge of phyllosilicate minerals using the Mular-Roberts titration technique. Minerals Engineering, vol. 23. pp. 383-389. [ Links ]
Araujo, A.C., Viana, P.R.M., and Peres, A.E.C. 2005. Reagents in iron ores flotation. Minerals Engineering, vol. 18. pp. 219-224. [ Links ]
Astrup, J., Hammerbeck, E.C.I., and van den Berg, H. 1998. Iron. The Mineral Resources of South Africa. Wilson, M.G.C. and Anhaeusser, C.R. (eds.). Handbook 16, Council for Geoscience, Pretoria. pp. 402-416. [ Links ]
Fuerstenau, D.W. and Pradip. 2005. Zeta potentials in the flotation of oxide and silicate minerals. Advances in Colloid and Interface Science, vol. 114-115. pp. 9-26. [ Links ]
Sirkeci, A.A. 2000. Electrokinetic properties of pyrite, arsenopyrite and quartz in the absence and presence of cationic collectors and their flotation behaviour. Minerals Engineering, vol. 13, no. 10/11. pp. 1037-1048. [ Links ]
Turrer, H.D.G. and Peres, A.E.C. 2010. Investigation on alternative depressants for iron ore flotation. Minerals Engineering, vol. 23. pp. 1066-106. [ Links ]
Vieira, A.M. and Peres, A.E.C. 2007. The effect of amine type, pH, and size range in the flotation of quartz. Minerals Engineering, vol. 20. pp. 1008-1013. [ Links ]
Paper received May 2015
Revised paper received Nov. 2015














