Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.6 Johannesburg Jun. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n6a16
PAPERS OF GENERAL INTEREST
Selenium minerals and the recovery of selenium from copper refinery anode slimes
C. Wang; S. Li; H. Wang; J. Fu
School of Mining Engineering, University of the Witwatersrand, Johannesburg, South Africa
SYNOPSIS
Since it was first identified in 1817, selenium has received considerable interest. Native selenium and a few selenium minerals were discovered several decades later. With the increasing number of selenium minerals, the occurrence of selenium minerals became the focus of much research. A great number of selenium deposits were reported all over the world, although few independent selenium deposits were discovered. Selenium is obtained mainly as a byproduct of other metals, and is produced primarily from the anode mud of copper refineries. This paper presents a comprehensive review of selenium minerals, as well as the treatment of copper refinery anode slimes for the recovery of selenium. Our focus is on the selenium minerals, including their discovery and occurrence, and the distribution of selenium resources. In addition, the main methods of recovering selenium from copper anode slimes are summarized.
Keywords: selenium, selenium minerals, anode slimes
Introduction
Selenium (Se) was first observed in 1817 in a laboratory (Greenwood et al., 1984). The discovery of selenium was made by the Swedish chemist J.J. Berzelius and J.G. Gahn, who isolated selenium from a red residue in sulphuric acid from pyrite mined at Fahlun, Sweden. Selenium was named from the Greek word selene (moon), since it resembled tellurium, which had been discovered a few years earlier and named from the Latin word tellus (Earth).
After the first observation of selenium in the laboratory, selenium received considerable interest. In 1954, E.P. Kaiser pointed out that Se is enriched in sulphide ores and often associated with Bi, Co, Sn etc. (Kaiser 1954). In 1959, Hawley and Nichol investigated selenium in Canadian sulphide minerals, and presented the content of selenium in sulphide from several deposits of different types. They also proposed that Se is enriched in low-temperature hydrothermal pyrite (Hawley and Nichol, 1959). The thermodynamic conditions for forming native selenium and selenium minerals in sedimentary rocks were discussed, as well as geochemical behaviour of selenium near the oxidation zones of sulphides in the 1970s (Howard III, 1977; Zhu et al., 2003). Zhu et al. examined the morphology, features, and genesis of native selenium from Yutangba, Enshi City, Hubei Province, China in 2004, and pointed out, from the different forms of native Se, that selenium can be activated,transformed, remobilized, and enriched at sites such as in the unsaturated subsurface zone or in the saturated zone (Zhu et al., 2005). The transport and deposition of selenium in felsic volcanic-hosted massive sulphide deposits of the Yukon Territory, Canada was studied and reported by Layton-Matthews et al. (2005).
Selenium is a comparatively rare and greatly dispersed element. The average selenium content in the Earth's crust is considered to vary between 0.05 and 0.09 μg/g (Lakin 1972; Greenwood et al., 1984; Jiajun et al., 1997). Elemental selenium is seldom found in nature; industrially, selenium is obtained as a by-product of mining other metals such as copper, iron, and lead (Fishbein 1983; Wen and Qiu 1999). It is produced primarily from the anode slimes of copper refineries (Butterman et al., 2004). There are various reports in the literature on the treatment of copper anode slimes to recover selenium (Hoffmann 1989; Cooper 1990).
In this paper, we provide an overview of selenium minerals, as well as the recovery of selenium from copper refinery anode slimes. Our focus is on the occurrence of selenium minerals and selenium deposits. We also examined the research work reported in the literature on the treatment of copper refinery anode slimes for the recovery of selenium.
Selenium minerals
In 1956, Thompson et al. discovered felty native selenium, which is violet acicular crystal. Coleman and Delevaux (1957) investigated the occurrence of selenium in sulphide from sandstone-type uranium ores in the western USA, and discovered trigonal and monoclinic native selenium, as well as clausthalite and ferroselite (Thompson et al,. 1956; Coleman and Delevaux, 1957). The occurrence of large particle of native selenium up to 20-30 mm in length in coal seams was reported by Zhu et al. (2005). In 1990, spherical and tubular native selenium was discovered in a hydrothermal U-Se-Re polymetallic deposit (Zhu et al., 2003).
The selenide deposits in the eastern Harz Mountains, Germany were intensively studied. The selenide-bearing deposits at Tilkerode, Lerbach, Clausthal, Zorge, St. Andreasberg, and Trogthal, are recognized as typical selenide vein deposits of telethermal origin (Simon et al., 1997). The polymetallic selenide mineralization at Tilkerode, Zorge, Lerbach, and Trogthal occurs mainly as small deposits not associated with larger base-metal deposits, while the selenide minerals at Clausthal and St. Andreasberg are associated with larger deposits mined primarily for silver, lead, zinc, and copper. The series of selenides from Tilkerode was identified mainly by Tischendorf: clausthalite (PbSe), naumannite (Ag2Se), tiemannite (HgSe), eskebornite (CuFeSe2), trogtalite (CoSe2), hastite (CoSe2), freboldite (CoSe), bornhardtite (Co3Se4), berzelianite (Cu2Se), umangite (Cu3Se2), klockmannite (CuSe), and stibiopalladinite (Pd5Sb2) (Davis et al., 1977; Stanley et al., 2002). Two unidentified minerals, noted by Tischendorf in 1958, represented two new species: chrisstanleyite (Ag2Pd3Se4) and tischendorfite (Pd8Hg3Se9) (Stanley et al., 1990, 2002). Wallis described a number of additional mineral species in the Tilkerode selenide assemblage: krü taite (CuSe2), athabascaite (Cu5Se4), temagamite (Pd3HgTe3), eucairite (CuAgSe), trüstedtite (Ni,Se4), penroseite ((Ni,Co,Cu)Se2), and geffroyite ((Ag,Cu,Fe)9(S,Se)8) (Stanley et al., 2002).
Davis et al. (1977) reported a new selenide of palladium, palladseite (Pd17Se15), which occurs in the residual concentrates from gold washing at Itabira, Minas Gerais, Brazil and is associated with arsenopalladinite, isomertieite, and atheneite.
Selenian miargyrite (AgSb(Se06S14)2), a new variety of miargyrite, was found in gold-bearing quartz veins in middle-lower Silurian quartzite of the Ailaoshan metamorphic belt, Yunnan province, China. It is intimately associated with freibergite, stibnite, ullmannite, and native gold and occurs as grains from 0.1 to 0.02 mm in diameter (Yunfen et al., 1990).
Stanley et al. (1990) examined the precious and base metal selenide mineralization at Hope's Nose, Torquay, Devon, England. The selenide assemblage consists of clausthalite (PbSe), tiemannite (HgSe), klockmannite (CuSe), umangite (Cu3Se2), tyrrellite ((Cu,Co,Ni)3Se4), trustedtite (Ni3Se4), penroseite (NiSe2), naumannite (Ag2Se), eucairite (AgCuSe), and fischesserite (Ag3AuSe2). A new mineral, chrisstanleyite (Ag2Pd3Se4), was discovered in gold-bearing carbonate veins in Middle Devonian limestones at Hope's Nose. It is associated with palladian and argentian gold, fischesserite, clausthalite, eucairite, tiemannite, umangite, cerussite, calcite, and bromian chlorargyrite (Paar et al., 1998).
Selenio-sulfantimonide was discovered in the Laerma gold-copper-uranium deposit in China in 1993. It is associated with native gold, tiemannite, clasthalite, lautite, aurostibite, gersdorffite, quartz, barite, etc. and occurs as fine grains, about 0.01-0.5 mm (Minghua et al., 1993).
As many selenium minerals were identified and discovered, numerous investigators conducted imvestigations on selenium minerals. More than 90 selenium minerals had been identified by 1998. Wen and co-workers reviewed the selenium minerals and their occurrence (Huayun, 1998).
Recently, a new series of selenium minerals was discovered. Stanley et al. (2002) reported a new mineral species from the Eskaborner Stollen at Tilkerode, Harz Mountains, Germany. Tischendorfite occurs as aggregates in a carbonate matrix, together with the associated metallic minerals clausthalite, tiemannite, chrisstanleyite, stibiopalla- dinite, and gold.
Schlemaite, a new mineral species from the Niederschlema-Alberoda vein-type uranium deposit, was discovered at Hartenstein, Erzgebirge, Germany. It occurs in aggregates of up to several hundred micrometres across, with berzelianite, eucairite, and clausthalite in a dolomite-ankerite matrix. Of the three vein-type uranium deposits in the Schneeberg-Schlema-Alberoda ore district, Niederschlema-Alberoda is considered the major occurrence of selenides in the Erzgebirge of Germany. In addition, rare selenides of Cu, Bi, Hg, Ni, and other elements were reported (Forster et al., 2003).
Jaguéite, the copper analogue of chrisstanleyite, was discovered in a telethermal selenide vein-type deposit at the El Chire prospect, Los Llantenes District of La Rioja Province, Argentina. The new species is generally associated with chrisstanleyite, particularly in intimate intergrowths, clausthalite, naumannite, tiemannite, klockmannite, berzelianite, umangite, and aguilarite. In addition, two unnamed compounds, chemically (Ag, Cu)6Hg2Pb2Se3 and (Ag, Cu)8Hg3(S, Se)7, occur as rare constituents (Paar et al., 2004).
Jolliffeite, previously known only from Lake Athabasca, Saskatchewan, Canad was reported from the Niederschlema-Alberoda uranium deposit in the Erzgebirge region of Germany. Jolliffeite, an exotic mineral, is associated with haematite, Ni-Co-Se-bearing lollingite, clausthalite, tiemannite, mercurian hakite-giraudite solid solutions, sulphurian berzelianite, sulphurian umangite, hessite, Ni-Co-As-bearing pyrite, and Se-rich chalcopyrite (Forster et al., 2004).
Three occurrences of clausthalite were reported in Poland, in abandoned polymetallic deposits at Kowary and Kletno and the Fore-Sudetic copper deposits (Thompson et al., 1956; Kucha, 1982). A new occurrence of clausthalite, together with uraninite, was reported in the Sudetes, southwest Poland. Clausthalite forms veinlets in a breccia comprising <50% calc- silicate rock fragments (Thompson et al., 1956).
Plumboselite, a new selenite from the Tsumeb mine, Namibia, occurs as fibres on clausthalite and is associated with smithsonite, mimetite, and vaterite. It occurs in subparallel to divergent clusters of thin, flattened, colourless fibres up to 0.3 mm in length (Kampf et al., 2011).
All the reported selenium minerals are shown in Tables I-III. The minerals are mainly selenides, selenium sulphides and oxygen-containing selenides (Huayun, 1998). There are a few native selenium minerals, selenium oxides, as well as intermetallic compounds between selenium and metals.
Selenium is chemically very similar to sulphur, and sulphur is the primary accompanying element in selenium minerals. Many of its compounds are analogues of sulphur compounds, and selenium substitutes for sulphur in minerals and other compounds.
Cu, Bi, Pb, Ag, and Te are the main element in selenium minerals, followed by Co, Ni, Fe, and Sb. Based on the content of selenium in sulphides, Yang (Huayun, 1998) described the affinity between Se and related elements, which can be divided into three categories (with decreasing affinity): (1) Pb, Ag, Bi, Hg, Cu; (2) Co, Ni; (3) Fe, Zn. Se forms a few minerals with precious metals, e.g. Au, Ag, Pt, Pb.
Mineralogical studies of numerous selenium minerals have not been fully conducted due to their restricted occurrence, small particle size, and experimental limitations. For instance, there are five selenides of nickel: kullerudite, makinenite, sederholmite, trustedtite, and wilkmanite, but no information has been reported about reflectivity or color index (Huayun, 1998).
Occurrence of selenium minerals
Selenium minerals occur in various forms, which can be divided primarily into three categories: independent minerals; isomorphism; adsorbed on clay minerals. Although there are about 100 selenium minerals, the metal rarely occurs in commercial concentrations. Selenium occurs mainly in sulphides or sulphosalt minerals in the form of isomorphism; there are rare selenium deposits that are commercially viable (Brown Jr, 1998; Huayun, 1998).
Selenium deposits can be divided into independent deposits and accompanying deposits. The occurrence of some selenium minerals is shown in Table IV. As regards independent selenium deposits, hydrothermal deposits are dominating type, and the Pakarharkar deposit in Bolivia is typical, while the Yutangba Se deposit in China, a sedimentary deposit, is an exception.
Selenium resources occur mainly in combined deposit, which can be divided into several industrial types, i.e. magmatic, porphyry, skarn, hydrothermal, volcanogenic-sedimentary, and sedimentary. Among these, magmatic, porphyry, hydrothermal, and sedimentary type deposits are dominant.
Selenium minerals are often closely associated with other minerals in very fine particles. For example, clausthalite occurs mainly as finer particles in the size range from 0.005 mm to 0.01 mm in the Wolverine deposit, Yukon Territory, Canada. It occurs with sulphides and is often associated with tetrahedrite or silver stibnic clausthalite in the same grain (Figure 1).

The mechanism of selenium mineral formation remains unclear. Selenium minerals are often associated with gold, but no selenium-gold minerals have been discovered. Furthermore, the relationship between selenium and sulphur in minerals is still indistinct, e.g. the replacement of selenium is ordered or disordered.
Distribution of selenium resources
According to the US Bureau of Mines (USBM), the world's selenium reserve base is 1.34x1051, and the proved reserves amount to 7.1x1041 (Brown Jr, 1998). The reserves are dominated by America with 52.7% of the total reserves; Asia, Africa, Europe, and Oceania account for 15.4%, 15.4%, 12.2%, and 4.4%, respectively (Feng and Jiajun, 2002). Chile, America, Canada, China, Zambia, Zaire, Peru, the Philippines, Australia, and Papua New Guinea account for about 76.9% of the proved reserves. There are about 40 countries that lack selenium resources.
Independent industrial deposits of selenium are rare. No independent selenium deposits were reported until the discovery of the Pakarharkar deposit in Bolivia in the 1980s. Since then, a series of associated deposits have been discovered in Canada, America, Chile, Zambia, Zaire, etc. (Daming, 1996). The main Se deposits of the world are shown in Table V.
Recently, selenium resources were reported at the Wolverine and KZK deposits in the Finlayson Lake District (FLD) of the Yukon, Canada. In the mid-1990s, three polymetallic volcanic-hosted massive sulphide deposits were discovered in the FLD with a combined resource of 21.5 Mt. Elevated selenium levels in the Wolverine and KZK massive sulphide ores were recognized during metallurgical testing and in the pre-feasibility stages of exploration. Selenium concentrations up to 3420 g/t are reported, with a mean value of approximate 700 g/t (Layton-Matthews et al., 2005). The selenium in the Wolverine deposit occurs mainly as fine particles of clausthalite and silver stibnic clausthalite between 0.005-0.05 mm in diameter.
China is one of the major countries that hold selenium resources, with the fourth-largest recoverable reserves after Canada, America, and Belgium (Brown Jr, 1998). There are 10 ascertained Se deposits, including Jinshan gold deposit, Baijiazuizi Cu-Ni deposit, and Chengmenshan copper deposit (Zuomin, 1997). In addition, some independent selenium deposits have been discovered in China, i.e. the Yutangba Se deposit and Laerma Se-Au deposit (Wen and Qiu, 1999). Proven reserves of selenium occur mainly with copper and nickel ores, and are distributed in the northwest of China and the Lower Yangtze region. The main Se deposits of China are shown in Table VI.
The recovery of selenium from copper anode slimes
The largest source of selenium is the anode slimes formed during the electrolytic refining of copper (Elkin and Margrave, 1982). Production usually begins by oxidation to produce selenium dioxide at an appropriate temperature. The selenium dioxide is then dissolved in water and the solution is acidified to form selenous acid (oxidation step). Selenous acid is sparged with sulphur dioxide (reduction step) to yield elemental selenium. In South Africa, Se is mostly removed to about 1 mg/L before the leach solution is sent to copper electrowinning. In South African refineries, Se is recovered mostly by precipitation using sulphurous acid. Different approaches to treating anode slimes are shown in Figure 2.

Sulphatizing roast
In the sulphatizing roast, sulphuric acid is used as an oxidant in the presence of air for the conversion of selenium or selenides to their tetravalent oxides (Hoffmann et al., 1976; Hoffmann, 1989). Selenium is volatilized as selenium dioxide and passes into the scrubbers. The scrubbing of the off-gases results in complete recovery of the selenium from the gas stream. The process is shown in Figure 3.
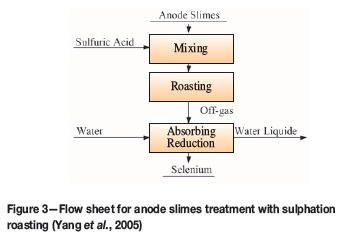
Although the process entails an advantage in that the sulphur dioxide produced in the roasting step reduces the selenious acid to elemental selenium and the sulphuric acid consumed in the roast is regenerated, this process is a net consumer of sulphuric acid. In addition, the rather long reaction times required for the oxidation of the selenium, extensive foaming due to sulphur dioxide liberation, as well as the cumbersome equipment due to large allowances for freeboard add difficulties to the process.
Soda roast
Slimes are mixed with sodium carbonate, a binder clay, and water to form a stiff paste, and then pelletized, dried, and roasted at a low temperature (530-650°C) to convert all selenium and tellurium to the soluble hexavalent state. The roasted pellets are ground and leached in water. Selenium goes into solution (as the selenate Na2SeO4), while tellurium is insoluble in the alkaline solution; thus the two elements are separated in this stage (Figure 4).

Two processes are commonly employed for the reduction of hexavalent selenium from solution (Figure 5) (Hoffman 1997; Yang et al. 2005). In the first process, selenium is leached from the slimes and recovered from solution by crystallization. The crystalline sodium selenate is mixed with charcoal and reduced to sodium selenide under controlled conditions of heating. The sodium selenide cake is leached with water, and the dissolved sodium selenide is readily oxidized to the elemental form by blowing air through the solution. The recycling of much of the solution is a significant advantage considering the severe restrictions placed on the discharge of selenium-bearing solutions.

In the other process, the hexavalent selenium is reduced using concentrated hydrochloric acid or ferrous iron salts catalysed by chloride ions as the reductant. The process generates large volumes of ferric chloride effluent, which is extremely corrosive and problematic to discharge.
Oxidizing roast
Oxidation roasting of anode slimes can be employed to eliminate selenium following sulphuric acid leaching of the slimes (Morrison, 1977; Hyvarinen, et al., 1984). Anode slimes are roasted to convert selenium to selenium dioxide, which reacts with water to form selenic acid in the dust collector. The selenic acid is reacted with copper powder, carbon (soot), and SO2 in the furnace gas, and it is reduced to selenium or insoluble selenide. The process is shown in Figure 6.

Chlorination processes
The chlorination of anode slimes has been the focus of considerable research (Hyvarinen et al., 1984). Both wet and dry chlorination process have been developed. Dry chlorination processes have not proven to be industrially viable, while wet chlorination of refinery slimes is a rapid and simple method with high extraction of selenium from slimes. The wet chlorination process may involve changing all of the process chemistry, not just the removal of selenium (Hoffmann, 1989).
Wet chlorination of slimes involves sparging slimes slurried in water or hydrochloric acid with chlorine gas at about 100°C to oxidize and dissolve selenium and selenides. The selenium in solution may be converted initially to the hexavalent state by chlorine, and then reduced to the tetravalent state as the pH decreases. Selenium can be reduced selectively from the chlorination liquor by sulphur dioxide.
Conclusion
Since the first observation of selenium in the laboratory, more than 100 selenium minerals have been discovered and reported. The minerals are mainly selenides, selenium sulphides, and oxygen-containing selenides. Sulphur is the primary element in selenium minerals as a consequence of its chemical similarity with selenium, while Cu, Bi, Pb, Ag, and Te are the other main elements, followed by Co, Ni, Fe, andSb.
Selenium deposits can be divided into independent deposits and accompanying deposits. A few independent selenium deposits have been reported, such as the Pakarharkar deposit in Bolivia and Yutangba Se deposit in China. Se resources occur mainly in combined deposits.
Accompanying deposits can be divided into several genetic types, with magmatic, porphyry, hydrothermal, and sedimentary type deposits being dominant. Selenium minerals are often closely associated with other minerals in very fine particles in combined deposits. Although numerous selenium deposits have been discovered all over the world, there are few deposits that are commercially viable. Industrial production of selenium is mainly as a by-product of other metals, such as copper, iron, and lead. Selenium is produced primarily from the anode mud of copper refineries, and this process has been investigated widely.
In investigations of the selenium minerals, comprehensive mineralogical studies of numerous minerals have not been conducted due to their restricted occurrence, small particle size, and experimental limitations. Furthermore, the mechanism of selenium mineral formation remains unclear. Selenium minerals are often associated with gold, although no independent minerals consisting of selenium and gold have been discovered. The relationship between selenium and sulphur in minerals is still unclear.
References
Brown Jr, R.D. 1998. Selenium and tellurium. US Geological Survey, Arlington, VA. [ Links ]
Butterman, W.R. and Brown, R.D. 2004. Selenium. Mineral Commodity Profiles. US Department of the Interior, US Geological Survey, Arlington, VA. [ Links ]
Coleman, R.G. and Delevaux, M.H. 1957. Occurrence of selenium in sulfides from some sedimentary rocks of the western United States. Economic Geology, vol. 52, no. 5. pp. 499-527. [ Links ]
Cooper, W.C. 1990. The treatment of copper refinery anode slimes. JOM, vol. 42, no. 8. pp. 45-49. [ Links ]
Daming, P. 1996. Seldom selenium resource in China. China Non-metallic Mining Industry Herald (in Chinese) [ Links ]
Davis, R., Clark, A.M., and Criddle, A.J. 1977. Palladseite, a new mineral from Itabira, Minas Gerais, Brazil. Mineralogical Magazine, vol. 41, no. 123. pp. M10-M13. [ Links ]
Elkin, E. and Margrave, J. 1982. Selenium and selenium compounds. Kirk- Othmer Encyclopedia of Chemical Technology, vol. 20. Wiley, New York. pp. 575-601. [ Links ]
Forster, H.J., Cooper, M.A., Roberts, A.M., Stanley, C.J., Criddle, A.J., Hawthorne, F.C., Laflamme, J.H.G., and Tischendorf, G. 2003. Schlemaite, (Cu, )6(Pb,Bi)Se4, a new mineral species from Niederschlema-Alberoda, Erzgebirge, Germany: description and crystal structure. Canadian Mineralogist, vol. 41, no. 6. pp. 1433-1444. [ Links ]
Forster, H.J., Rhede, D., and Tischendorf, G. 2004. Mineralogy of the Niederschlema-Alberoda U-Se-polymetallic deposit, Erzgebirge, Germany. I. Jolliffeite, NiAsSe, the rare Se-dominant analogue of gersdorffite. Canadian Mineralogist, vol. 42, no. 3. pp. 841-849. [ Links ]
Feng, C. and Jiajun, L. 2002. An outline of selenium resources and its exploitation and utilization. Geology and Resources, vol. 11, no. 3. pp. 152-156 (in Chinese). [ Links ]
Fishbein, L. 1983. Environmental selenium and its significance. Fundamental and Applied Toxicology, vol. 3, no. 5. pp. 411-419. [ Links ]
Greenwood, N.N. and Earnshaw, A. 1984. Chemistry of the Elements. Butterworth-Heinemann. [ Links ]
Hawley, J.E. and Nichol, 1.1959. Selenium in some Canadian sulfides. Economic Geology, vol. 54, no.4. pp. 608-628. [ Links ]
Hoffman, J.E. and King, M.G. 1997. Selenium and selenium compounds. Encyclopedia of Chemical Technology. Wiley, New York. pp. 686-719. [ Links ]
Hoffmann, J.E. 1989. Recovering selenium and tellurium from copper refinery slimes. JOM, vol. 41, no. 7. pp. 33-38. [ Links ]
Hoffmann, J.E., Parker, P.D., and Sabo, A.C. 1976. Extraction and purification of silver from sulfates. US patent 3996046. Amax Inc. [ Links ]
Howard III, H. 1977. Geochemistry of selenium: formation of ferroselite and selenium behavior in the vicinity of oxidizing sulfide and uranium deposits. Geochimica et Cosmochimica Acta, vol. 41, no. 11. pp. 1665-1678. [ Links ]
Huayun, W.H.X. 1998. A review of selenium minerals. Acta Petrrologica et Mineralogia, vol. 17, no. 3. pp. 261-265 (in Chinese). [ Links ]
Hyvarinen, O. Rosenberg, E., and Lindroos, L. 1984. Selenium and precious metals recovery from copper anode slimes at outokumpu Pori Refinery. Precious Metals: Mining, Extraction and Processing. Kudryk, V., Corrigan, D.A., and Liang, W.W. (eds). AIME/TMS, The Minerals, Metals and Materials Society, Warrendale, PA. pp. 37-548. [ Links ]
Jiajun, L., Minghua, Z., JIANMING, L., Yufeng, Z., Xuexiang, G., Bin, Z., Li, L., and Wenquan, L. 1997. Selenium enrichment in Cambrian stratabound gold deposits in the western Qinling Mountains. Acta Geologica Sinica (English Edition), vol. 71, no. 4. pp. 423-432 (in Chinese). [ Links ]
Kaiser, E. 1954. Selenium in sulfide ores. Geological Society of America Bulletin, vol. 65. p. 1379. [ Links ]
Kampf, A.R., Mills, S.J., and Pinch, W.W. 2011. Plumboselite, Pb3 O2 (SeO3), a new oxidation-zone mineral from Tsumeb, Namibia. Mineralogy and Petrology, vol. 101, no. 1. pp. 75-80. [ Links ]
Kucha, H. 1982. Platinum-group metals in the Zechstein copper deposits, Poland. Economic Geology, vol. 77, no. 6. pp. 1578-1591. [ Links ]
Lakin, H.W. 1972. Selenium accumulation in soils and its absorption by plants and animals. Geological Society of America Bulletin, vol. 83, no. 1. pp. 181-190. [ Links ]
Layton-Matthews, D., Scott, S.D., P, J.M., and Leybourne, M.I. 2005. Transport and deposition of selenium in felsic volcanic-hosted massive sulfide deposits of the Finlayson Lake District, Yukon Territory, Canada. Mineral Deposit Research: Meeting the Global Challenge. Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18-21 August 2005. Mao, J. and Bierlein, F.P. (eds). Springer. pp. 643-646. [ Links ]
Zheng, M., Liu, J., Liu, J., and Lu, W. 1993. The first discovery and preliminary study of selenio-sulfantimonide in China. Journal of Mineralogy and Petrology, vol. 13, no. 2. pp. 9-13 (in Chinese). [ Links ]
Morrison, B.H. 1977. Slimes treatment process. US patent 4,047,939. Norand Mines Ltd. [ Links ]
Paar, W., Roberts, A.C., Criddle, J., and Topa, D. 1998. A new mineral, chrisstanleyite, Ag2Pd3Se4 from Hope's Nose, Torquay, Devon, England. Mineralogical Magazine, vol. 62, no. 411. pp. 257-264. [ Links ]
Paar, W.H., Topa, D., Makovicky, E., Sureda, R.J., de Brodtkorb, M.K., Nickel, E., and Putz, H. 2004. Jaguéite, Cu2Pd3Se4, a new mineral species from El Chire, La Rioja, Argentina. Canadian Mineralogist, vol. 42, no. 6. pp. 1745-1755. [ Links ]
Simon, G., Kesler, S.E., and Essene, E.J. 1997. Phase relations among selenides, tellurides, and oxides; II, Applications to selenide-bearing ore deposits. Economic Geology, vol. 92, no. 4. pp. 468-484. [ Links ]
Stanley, C., Criddle, A., and Lloyd, D. 1990. Precious and base metal selenide mineralization at Hope's Nose, Torquay, Devon. Mineralogical Magazine, vol. 54. pp. 485-493. [ Links ]
Stanley, C. J., Criddle, A.J. Forster, H-J., and Roberts, A.C. 2002. Tischendorfite, Pd8HgjSe9, a new mineral species from Tilkerode, Harz Mountains, Germany. Canadian Mineralogist, vol. 40, no. 2. pp. 739-745. [ Links ]
Thompson, M., Roach, C., and Braddock, W. 1956. New occurrences of native selenium. American Mineralogist, vol. 41. pp. 156-157. [ Links ]
Wen, H. and Qiu, Y. 1999. Organic and inorganic occurrence of selenium in Laerma Se-Au deposit. Science in China Series D: Earth Sciences, vol. 42, no. 6. pp. 662-669 (in Chinese). [ Links ]
Yang, C., Zhang, X., and Lan, D. 2005. Present situation and trend of selenium removal technique from copper anode slime. Sichuan Nonferrous Metals, vol. 1. pp. 22-25 (in Chinese). [ Links ]
Yunfen, L., Yueying, L., Mingming, Y., Kuishou, D., Naijue, Z., and Fang, G. 1990. Selenian miargyrite-a new variety of miargyrite. Geological Review (in Chinese). [ Links ]
Zhu, J., Liang, X., Qang, M., Wang, F., Ling, H., and Liu, S. 2003. Advances in studying occurrence modes of selenium in environment. Bulletin of Mineralogy, Petrology and Geochemistry, vol. 22, no. 1. pp. 75-81 (in Chinese). [ Links ]
Zhu, J. and Zheng, B. et al. 2005. Morphology features and genesis of native selenium. Bulletin of Mineralogy, Petrology and Geochemistry, vol. 19, no. 4. pp. 353-355 (in Chinese). [ Links ]
Zuomin, H. 1997. Aulacogens of middle late Proterozoic period along the northern margin of North China Platform. Geology of Chemical Minerals, vol. 1 (in Chinese). [ Links ] ♦
Paper received Dec. 2012
Revised paper received Feb. 2016.














