Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.6 Johannesburg Jun. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n6a12
PAPERS - COOPER COBALT AFRICA CONFERENCE
Unlocking Rustenburg Base Metals Refiners sulphur removal section
J. HagemannI; M. PelserII
IAnglo American Platinum Ltd, Rustenburg, South Africa
IIAnglo American Technical Solutions, Johannesburg, South Africa
SYNOPSIS
The Anglo American Platinum Rustenburg Base Metals Refiners (RBMR) is the largest producer of nickel cathode in South Africa. It treats slow-cooled matte to recover a platinum group metal-containing magnetic fraction for downstream processing and a nonmagnetic fraction to produce nickel and copper cathode, cobalt sulphate, and sodium sulphate. The refinery was recently expanded from 21 to 33 kt/a nickel. During the ramp-up phase, the sulphur removal section was found to be a constraint to throughput. Nickel hydroxide is precipitated from nickel spent electrolyte by addition of sodium hydroxide, and filtration of the nickel precipitate allows sulphur to be removed from the circuit as aqueous sodium sulphate in the filtrate. The filtration fluxes were found to be limiting the circuit's capacity. An obvious solution would have been to install additional filtration capacity at significant capital cost and long lead times. RBMR chose rather to first initiate a collaborative project with Anglo American Technical Solutions to better understand the impact of process conditions on filtration characteristics and to evaluate whether the filtration flux could be increased by improving the quality of the nickel precipitate.
A deeper understanding of nickel hydroxide precipitation chemistry and kinetics in the context of high nickel and sodium hydroxide concentrations was developed, guided by precipitation theory and confirmed by laboratory tests and plant observations. It was found that the filtration rate could be significantly improved by modifying the precipitate characteristics. A new precipitation process was engineered and retrofitted into the existing plant. Since start-up of the new precipitation system, the available capacity of the sulphur removal section has more than doubled, and control of the process has improved with the better agitation. This paper describes the theory and results used to design the new agitation system, as well as the impact of the change on capital investment, operability, and process efficiency.
Keywords: base metal refining, nickel hydroxide, precipitation, filtration, agitation, sulphur removal
Introduction
The Anglo American Platinum Rustenburg Base Metal Refiners (RBMR) forms part of the company's smelting and refining complex in Rustenburg for separating and recovering platinum group metals (PGMs) as well as base metals. The hydrometallurgical route for treating converter matte was adopted by Anglo American Platinum in 1966. Since its inception the refinery has undergone a number of expansions, including the commissioning of a new refinery in 1981 and an expansion to 33 kt/a nickel in 2011. The process has evolved to increase throughput, increase recovery, and improve the safety, health, and environment aspects of the operation. The 2011 brownfield expansion entailed changes to the process chemistry and technology using the existing assets with minimal additional equipment. The major new equipment included a new tankhouse for nickel electrowinning. The changes are described in detail by Bryson et al. (2008). The expansion project proceeded in a capital-constrained environment following the 2008 financial crisis. The project itself was deferred for a year in 2009 and the plant started plating cathode from the new tankhouse only in 2011. Technical difficulties in integrating certain of the new processes and equipment prolonged the ramp-up phase for the plant (Anglo American Platinum Limited Annual Report, 2012). The sulphur removal section in the BMR was found to be a constraint to throughput in 2013, preventing the plant from reaching its nameplate capacity.
Nickel hydroxide is precipitated from nickel spent electrolyte by addition of sodium hydroxide, and filtration of the nickel precipitate removes sulphur from the circuit as aqueous sodium sulphate in the filtrate. The hydroxide is retained within the circuit by redissolution using additional nickel spent electrolyte. This section was identified as a bottleneck by the frequent build-up of solution ahead of the processing step to a point where the plant needed to turn down or stop. The rate of flow is dictated by the flux achievable during the filtration of the hydroxide precipitate. The nominal throughput capacity of the section was estimated to be equivalent to 21 kt/a (the original plant capacity, even though additional filtration capacity had been installed as part of the expansion project) and needed to be increased by more than 50% to reach 33 kt/a.
The obvious solution to increase throughput was to install additional filtration capacity at significant additional capital expenditure. The capital requirement for expanding the filtration capacity was estimated to be in excess of R60 million with a lead time of 9 months for the first filter. RBMR chose rather to initiate a collaborative project with Anglo American Technical Solutions (ATS) to better understand the impact of process conditions on filtration characteristics and to evaluate whether the filtration flux could be increased by improving the quality of the nickel precipitate. Underpinning the project are the fundamentals of precipitation (nucleation and growth mechanisms), which are constrained by the desired chemistry of the overall circuit. The scope of the investigation included generating a deeper understanding of the processes involved during nickel hydroxide precipitation in the context of high nickel and sodium hydroxide concentrations; quantifying the extent to which filtration flux can be improved by manipulating the precipitation conditions; and engineering of the process equipment capable of delivering the desired precipitate.
Sulphur deportment
The RBMR flow sheet can be simplified as shown in Figure 1 for purposes of discussing the sulphur balance. The slow-cooled matte, better known as Waterfall Converter Matte (WCM), is received by RBMR where it undergoes a number of milling and magnetic separation steps to produce a nonmagnetic nickel-copper matte (NCM) and a magnetic-enriched concentrate (MEC). The sulphur associated with the matte accounts for 70% of the total sulphur input into the refinery. The MEC is subjected to a number of oxidative pressure leaching and atmospheric leaching steps targeting the base metal phases to produce a PGM-rich residue. Sulphuric acid is used as lixiviant in these leaching steps, accounting for the additional sulphur input.
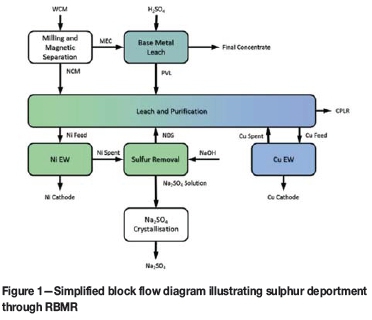
The NCM undergoes a series of metathetic and oxidative leaching steps in the leach and purification section to solubilize and separate nickel from copper. Sulphur is solubilized by the oxidation of sulphides to sulphate. The liquors undergo further purification, including the recovery of cobalt as well as the removal of selenium, tellurium, iron, lead, and zinc. The sulphur-based reagents used in this section add to the total sulphur input into the refinery.
Not all of the sulphur in the matte is solubilized and the remaining sulphur is rejected in the copper pressure leach residue (CPLR). The amount of sulphur rejected with the residue depends on the extent of sulphur oxidation during copper leaching. The solubilized sulphur passes through the copper electrowinning tankhouse to produce copper spent electrolyte. It is used to replace fresh acid addition into the leach and purification section and migrates through the circuit to form part of the feed stream to nickel electrowinning. Some sulphur is rejected from the circuit during the various purification steps, while the remaining bulk of the sulphur passes through the nickel electrowinning tankhouse to be rejected in the sulphur removal section. Nickel is returned to the leach and purification section as nickel dissolution solution (NDS). The sodium sulphate produced from the sulphur removal section is transferred to the sodium sulphate crystallization section for recovery. This results in the removal of an estimated 60 kt/a sodium sulphate through the sulphur removal section at an equivalent production rate of 33 kt/a nickel cathode.
Sulphur removal
Soluble sulphur is rejected by the filtration of nickel hydroxide from neutralized nickel spent electrolyte. The sulphur removal section (Figures 2 and 3) draws spent electrolyte from storage into the leading reactor, neutralizing free acid using sodium hydroxide. The nickel hydroxide is precipitated in the second reactor by raising the pH, again using sodium hydroxide. The reactors operate at 80°C. The volume of the reactors results in a mean residence time of approximately 30 minutes per reactor. The precipitated slurry is transferred through a pumpbox to a bank of six rotary vacuum drum filters (Eimco filters). The resultant filtrate passes through a polishing filtration stage, raising the pH value to ensure the nickel concentration is below 10 mg/L before it is transferred to the crystallization plant. The hydroxide cake is repulped and transferred to the dissolution reactors where it is dissolved using additional spent electrolyte. The net result of precipitation and dissolution is the neutralization of free acid produced during electrowinning. The sodium hydroxide consumption therefore reflects nickel production. The dissolution solution is finally returned to the leach and purification section.
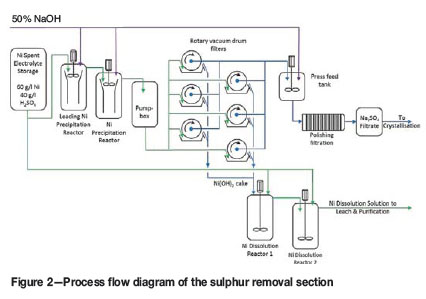

The titration curve of nickel spent electrolyte (Figure 4) indicates the initial neutralization of free acid followed by the precipitation of nickel hydroxide. The prominent pH increase between acid neutralization and nickel precipitation allows the two reactions to be segregated into the leading and precipitation reactors, respectively. The ratio of sodium hydroxide used for the two reaction steps depends on tankhouse operation (advance electrolyte tenor and bite) and is reflected in the split of spent electrolyte to precipitation and dissolution. This split ratio is not explicitly set during the operation of the plant. The operator sets the flow to precipitation and allows the controller to draw the spent required for dissolution based on a fixed pH set-point. The reactions are therefore inherently balanced.
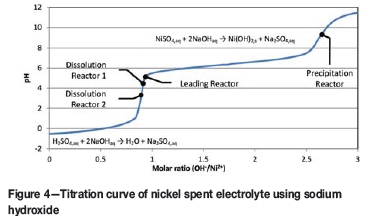
Ideally, the operator would control the flow to sulphur removal to balance nickel spent volume. This is, however, not possible in a system that is constrained by filtration capacity. The plant assumes the characteristics of a 'pull' system. The rotary vacuum drum filters operate at maximum capacity and the operator adjusts the throughput in discrete increments to the number of filters online. The pumpbox acts as a small buffer, allowing the operator to balance the flows. The flow is subject to fluctuations, causing instability in pH control which will be shown later in the paper to be severely detrimental to the filtration characteristics of the precipitate produced. This further aggravates the problem, causing the throughput to drop below that required to balance the spent electrolyte volume and for the storage tanks to fill. The last resort available to the operator is to drain the boots of the filters to the dissolution reactors, sustaining flow through the plant at the cost of retaining sodium sulphate in the circuit. This causes the sodium sulphate concentration to rise and solution storage in other parts of the plant to fill. Inevitably, the plant needs to be turned down or even stopped to allow the sulphur removal section to recover. The obvious solution to this problem is to increase the filtration capacity of the section through further capital investment. A more challenging solution involves re-evaluating the fundamental filtration characteristics of the nickel hydroxide precipitate, however unlikely this may seem since the existing circuit had operated for more than 30 years.
Methods of improving precipitate quality
Supersaturation is the extent by which a compound exceeds its theoretical solubility in solution, and impacts the properties of solids produced. As an example, Sõhnel and Garside (1992) discuss the relationship between supersatu-ration and precipitation. The control of supersaturation is not easy, given the low solubility levels and fast reaction kinetics involved during precipitation. The kinetics can exceed the rate of mixing, causing the precipitation reaction to be localized within the reactor volume. The concentration of the reactants and method of mixing play pivotal roles in the level of supersaturation generated and hence the quality of the precipitate produced.
Demopoulos (2009), in his review paper on precipitation in hydrometallurgy, suggests the following methods for controlling supersaturation:
pH control
Metal complexation and dissociation
Dilution
Redox reactions
Dissolution reactions.
The latter two options are not applicable to the large-scale production of nickel hydroxide. The redox method can be used only in multivalent systems such as Fe2+/Fe3+, while the dissolution method is complicated by the requirement to form a precursor compound from which the final precipitate is produced.
The method of supersaturation control via pH control was studied by Sist and Demopoulos (2003) on synthetic nickel laterite leach solutions (6 g/L Ni). It entails a semi-batch stepwise precipitation procedure, keeping the pH value below the point where the onset of homogeneous nucleation is observed. The method requires seed material to promote heterogeneous nucleation. According to the authors, the method can be translated to a continuous process where each step represents a reactor in series and the final product is recycled as seed. Application of this method at RBMR would require more than the suggested four stages, given the higher nickel concentration (60 g/L Ni).
The control of supersaturation via complexation during nickel hydroxide precipitation is achieved using ammonia, as described by Mubarok and Lieberto (2013) and E et al. (2015). The ammonia complex ((Ni(NH3)6)2+ lowers the concentration of free nickel, limiting the supersaturation level. A molar ratio NH4+:Ni2+ in excess of three is required for this method to work. The high ammonia concentration required excludes this method from being applied to RBMR.
Dilution of the reagent streams using barren solutions is a guaranteed method of reducing supersaturation, but it may not always be practical to apply. The extent of dilution achieved by addition of water or recycling of filtrate within the constraints of the process would be insufficient to alter the dominating precipitation mechanism. It is, however, possible to internally dilute the feed streams using the reactor's content, depending on the reactor's operating parameters and mixing. This proved to be the optimum solution for RBMR and will be discussed in more detail.
Investigation
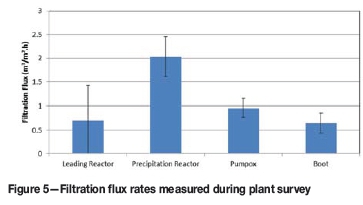
A plant survey was conducted in September 2013 to characterize the circuit. The filtration flux rates (flow of filtrate per unit filtration area) of samples from different points in the circuit were measured using a standardized filtration test (Figure 5). The filtration test was designed to simulate the rotary vacuum drum filters, targeting a similar cake thickness at the same vacuum pressure. Some solids were observed in the leading reactor and, in spite of the low solids loading, presented a high filtration resistance. The highest flux rates were observed at the precipitation reactor, decreasing to half of that value in the pumpbox and ending at one-third of the original value at the boot of the filters. This was interpreted to be indicative of a fragile precipitate and seems to have been considered in the design of the original plant. The reactors are fitted with low-intensity agitators (<0.07 kW/m3) and draught tubes with no agitation in the pumpbox. At the time of the survey, the initial laboratory results indicated that an improved precipitate could be produced under high-intensity agitation (approx. 0.7 kW/m3) leading to better dispersion of the reactants. The immediate action taken was to lower the NaOH addition point from the surface to a point close to the impeller in the precipitation reactor. This resulted in an increase in nominal throughput capacity from an estimated equivalent of 21 kt/a to 24 kt/a nickel.
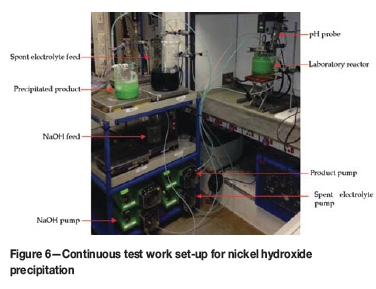
Investigation of the process continued at the hydromet-allurgy facilities of ATS. A laboratory reactor (Figure 6) was operated as a mixed suspension mixed product removal (MSMPR) reactor by continuously feeding plant solution and sodium hydroxide, and overflowing the nickel hydroxide product slurry. The reactor was baffled and agitated using a variable-speed overhead stirrer fitted with a double pitch-blade turbine impeller. The reactor was heated using thermal fluid to control the contents at 80±0.1°C. All feed and product vessels were placed on load cell platforms for mass balance purposes. A pressurized pH probe was used and the pH value was maintained within 0.05 pH units of the set-point by the controlled addition of 45% sodium hydroxide. All the equipment (pumps, stirrer, heating circulator, pH transmitter, and weight transmitters) were connected to a process controller for data logging and control purposes. The majority of the test work was conducted in a 2 L laboratory reactor. The effect of scale was evaluated in a 7.5 L laboratory reactor and a 50 L pilot plant reactor train. Scale-up was constrained by available equipment and mixing conditions could not be replicated in full.
The initial programme evaluated reaction conditions (agitation intensity, pH set-point, and residence time), reactor configuration (pre-neutralization, reagent dilution, reagent addition point, and seed recycle) and scale (2 L, 7.5 L, and 50 L). All the tests yield precipitates characterized by filtration flux rates more than double what was achieved under stable plant conditions. The results showed that the reaction condition had the most profound effect on the filtration characteristics of the precipitate. Little benefit was derived from changing the reactor configuration and the different scales evaluated showed the results to be scalable.
The improved results from the tests were explained as follows. The low solubility of nickel hydroxide and high concentrations of reactants (60 g/L Ni in spent electrolyte and 45-50% NaOH) can easily generate high supersaturation levels, pushing the system deep into the homogeneous nucleation region. Mixing in a continuous precipitation reactor involves contacting three fluids, consisting of the two reactant streams and the bulk solution. The combined concentration of the reactants (Ni and OH) in the bulk is kept at its lowest value by controlling the reactor close to the stoichiometric ratio. Supersaturation levels are then limited when mixing the feed streams with the bulk, effectively diluting the reactants prior to the reaction. This is easily achieved in a laboratory-scale reactor with high circulation rates promoting mixing of the inlet streams with the bulk.
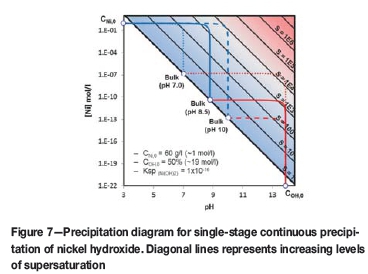
The mechanism can be further explained with reference to Figure 7. The black diagonal lines represent increasing levels of supersaturation starting from solubility (S = 1). The blue and red lines represent the mixing lines of spent electrolyte and sodium hydroxide with the bulk solution, respectively. The solid mixing lines represent the optimum pH value (approx. 8.5) with similar supersaturation levels generated while mixing the two reactants with the bulk. With a lower bulk pH value (7.0, dotted lines) the supersaturation level decreases for mixing the spent electrolyte, but increases for mixing sodium hydroxide. The opposite is true with a higher bulk pH value (10, dashed lines). The mixing line with the highest supersaturation level dominates, suggesting deteriorating filtration characteristics on both sides of the optimum.
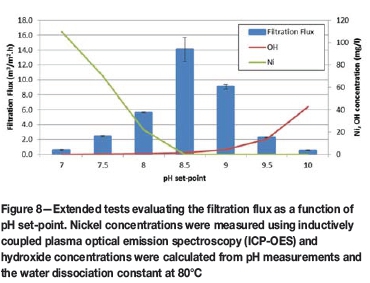
The explanation was tested against an expanded set of runs evaluating the pH set-point (Figure 8). The extents of precipitation in all of the tests were greater than 98%. The highest flux rate was achieved at the point of lowest combined reactant concentration. At pH set-points below this optimum point, the nickel concentration rises in the bulk solution, likely causing high supersaturation levels around the sodium hydroxide inlet. Again, at pH set-points above this optimum point, the hydroxide concentration rises in the bulk solution, likely causing high supersaturation levels around the nickel spent electrolyte inlet. The explanation held up to the extended test work and was used to develop the design intent of a new reactor.
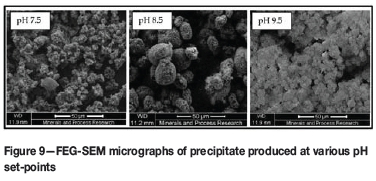
Field-emission gun scanning electron microscope (FEG-SEM) micrographs indicate a change in the habit of the precipitate formed (Figure 9). The shape of the precipitates produced at both high and low pH set-points tends to be highly irregular, forming loose agglomerates with some fine particles present. The precipitate produced at the optimum pH set-point is spherical in shape and more regular in size. The particle size distributions of the precipitates were measured using a laser diffraction instrument (Malvern Mastersizer). The Sauter mean diameters, d32, of the particles produced at both high (pH 9.5, 6.45 μΐη) and low (pH 7.5, 9.14 μπι) set-points were lower than at the optimum set-point (pH 8.5, 20.2 μm). Flux through a packed bed is proportional to the Sauter mean diameter of the particles, corroborating the results from the filtration tests.
Implementation
A new agitator was engineered and installed into the standby reactor in the precipitation train. The following design intent was used:
Single-stage precipitation of nickel hydroxide at optimum pH set-point
Promotion of mixing of feed streams with bulk solution
Increased circulation of the bulk to promote homogeneity.
The resulting design was a quad-impeller agitator with the feed streams added to the top and bottom of the reactor. Bulk mixing was realized through the multi-impeller arrangement while mixing of the feeds was realized at the individual top and bottom impellers. Offtake from the reactor is from the middle, which is also the pH measurement point. The installed cost of the new agitation system was less than R1 million.
The agitator was commissioned in June 2014. Mixing in the reactor was characterized prior to hot commissioning to confirm that it met the design intent. At the time of installation, there was still some uncertainty regarding the optimum agitation speed and the agitator was therefore fitted with a variable-speed drive. As part of commissioning, the circulation time of the reactor was measured at varying agitation speeds using the methods described by Bourne (1997). The agitation speed was selected to yield the design circulation time (6 seconds). Performance of the section has since been satisfactory, negating the need for further optimization. The measured residence time distribution approximated an ideal CSTR (continuous stirred tank reactor) while the circulation time was significantly lower compared to the old configuration (66 seconds).
Plant operation
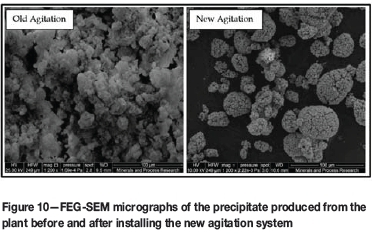
One of the immediate effects observed during commissioning was the lower boot levels in the rotary vacuum drum filters. The filtration characteristics of the precipitate improved to such an extent that the filtration rate exceeded the feed rate of the individual filters. Spot filtration tests suggest an improved, robust precipitate being produced, with filtration flux rates measuring up to 12.4 m3/m2.h at the reactor and decreasing by only 20% at the drum filter (67% historic). FEG-SEM micrographs (Figure 10) indicate that the habit of the precipitate had changed to match that observed in the optimum laboratory tests.
Operability and efficiency of the plant were assessed using process data for the period prior to the investigation, after the change in the NaOH addition point, and after the installation of the new agitation system. pH control in the reactor benefited from the improved dispersion of NaOH, resulting in a faster-responding signal. An advanced process controller was deployed with the new agitation system to help control pH. The overall result was a decrease in the standard deviation in the pH signal from 0.86 to 0.63 with the changed feed point, and finally to 0.48 with the new agitation system. The improved pH control is essential to consistently produce a good-quality precipitate, as was shown during the investigation.
Operability of the sulphur removal section is reflected in the storage levels of the spent electrolyte. The capacity of the storage is 670 m3, with intervention required as soon as the level reaches 600 m3. The frequency of spent storage exceeding 600 m3 has decreased from 9.1% to 4.8% with the changed feed point to 1.1% with the new agitation system at continually increasing throughputs. The typical number of filters online decreased from five to four with the new agitation system, while the mean boot levels dropped from 85% to 45%. The requirement to drain the filter boots has fallen away, resulting in the average sodium sulphate concentration of the nickel electrolyte decreasing from 170 g/L to 150 g/L. The sulphur removal section is no longer considered to be a constraint to throughput.
The sulphur removal section has consistently been able to match the plant production rate since the installation of the new agitation system. Two capacity runs were conducted to establish the new maximum capacity of the section. This was done by building spent electrolyte volume in storage tanks and then operating the plant with a reduced number of filters. The flow of spent electrolyte was systematically increased to the point where the pumpbox stabilized, indicating the filtration limit. The plant was maintained at this point until the spent volume was depleted. The equivalent nickel production rate was then calculated from the caustic consumption and extrapolated to an equivalent of five filters being online (five online, one standby). The two runs, first with one filter and the second with two filters, indicated an equivalent capacity of 49 and 55 kt/a, respectively. The section has sufficient filtration capacity to reach 33 kt/a, provided that the precipitate's filtration characteristics can be maintained during ramp-up.
Conclusions
The Anglo American Platinum Rustenburg Base Metal Refiners was recently expanded to 33 kt/a nickel cathode capacity and is in the process of ramping up. The sulphur removal section was found to be a constraint to throughput, limiting plant capacity. Through a collaborative effort with Anglo American Technical Solutions the sulphur removal section was unlocked from an equivalent capacity of 21 kt/a Ni to initially 24 kt/a Ni by moving the caustic addition point, and finally to a capacity well in excess of 33 kt/a Ni by installing a new agitation system. The new agitation system changed the habit of the precipitate to match that observed in the laboratory trials. The project was delivered from initialization through investigation and design to implementation in 12 months. The cost of installing the new agitation system was less than R1 million. This obviated the need of the fallback option, which would have required increasing filtration capacity by installing three additional rotary vacuum drum filters at an estimated cost of R60 million and with a lead time of 9 months for the first filter.
Acknowledgements
The authors would like to thank the management of Anglo American Platinum for permission to present this paper. This was a collaborative project with numerous individuals involved from ATS and RBMR. In particular, the authors would like to acknowledge the contributions of Nico Groenewald and Chandon Bezuidenhout on the test work; Gerhard van Wyk and the RBMR Engineering Department for the implementation of the agitation system; Shaun Johnson for the implementation of the advanced process controller; Eugene Els for the design of the agitation system; and Les Bryson for his support of the project.
References
Anglo American Platinum Ltd. 2012. Annual Report 2012. 276 pp. [ Links ]
Bourne, J.R. 1997. Mixing in single-phase chemical reactors. Mixing in the Process Industries. Harnby, N., Edwards, M.F., and Nienow, A.W. (eds.). Butterworth-Heinemann, Oxford. pp. 184-199. [ Links ]
Bryson, L.J., Hofrik, Z., Collins, M.J., Stiksma, J., and Berezowsky, R.M. 2008. New matte leaching developments at Anglo Platinum's base metal refinery. Hydrometallurgy 2008, Proceedings of the Sixth International Symposium.. Young, C.A., Taylor, C.A., and Choi, Y. (eds.). Society for Mining, Metallurgy and Exploration, Inc., Littleton, CO, USA. pp. 570-579. [ Links ]
Demopoulos, G.P. 2009. Aqueous precipitation and crystallisation for the production of particulate solids with desired properties. Hydrometallurgy, vol. 69. pp. 199-214. [ Links ]
Weiwei, E., Jingcai, C., Chao, Y., and Zai-Sha, M. 2015. Experimental study by online measurement of the precipitation of nickel hydroxide: Effects of operating conditions. Chinese Journal of Chemical Engineering, vol. 23. pp. 860-867. [ Links ]
Mubarok, M.Z. and Lieberto, J. 2013. Precipitation of nickel hydroxide from simulated and atmospheric-leach solution of nickel laterite ore. Procedia Earth and Planetary Science, vol. 6. pp. 457-464. [ Links ]
Sist, C. and Demopoulos, G.P. 2003. Nickel hydroxide precipitation from aqueous sulfate media. Journal of Metals, vol. 55, no. 8. pp. 42-46. [ Links ]
Söhnel, O. and Garside, J. 1992. Precipitation: Basic Principles and Industrial Applications. Butterworth-Heinemann, Oxford. [ Links ] ♦
This paper was first presented at the, Copper Cobalt Africa Conference, 6-8 July 2015, Avani Victoria Falls Hotel, Victoria Falls, Livingstone, Zambia.














