Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.6 Johannesburg Jun. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n6a11
PAPERS - COOPER COBALT AFRICA CONFERENCE
Review, evolution, and optimization of the treatment of Kansanshi mixed copper ore
C. Ngulube; C. Chongo; F.X. Paquot
First Quantum Minerals Ltd, Kansanshi Mine, Solwezi, Zambia
SYNOPSIS
Over the last six years, the Kansanshi copper-gold mine has continued to process significant quantities of complex transitional mixed ores. Initially, the ore treated came predominantly from accumulated stockpiles. Subsequently, the feed source shifted primarily to freshly mined ore that generally showed less tarnish with better flotation kinetics, but with the downside of lower concentrate grades. A number of changes were made to improve the efficiency of the rougher circuit and quality of concentrate produced. These changes were as a result of initial mineralogical studies and laboratory flotation test work that were discussed at length in a previous paper1. Salient among the recommendations adopted as a result of previous work were increased circuit capacity and a modified configuration to increase the residence time and the number of sulphidization stages. These modifications were completed and commissioned in 2011. This, however, resulted in significantly low rougher concentrate grades and a below-specification final concentrate product. This was due primarily to higher mass pulls from the extended rougher bank with multiple concentrate discharge streams, and which necessitated an enlarged cleaning capability. The extra cleaning capacity came on stream in 2014. Of note was the development of a recovery model, based on the strong correlation between the total copper and acid-soluble copper ratio, as a tool for monitoring plant performance.
Keywords: copper, flotation, optimization, mixed ore, sulphidization, modelling
Introduction
The Kansanshi mine, the largest copper mine in Africa, is 80% owned by Kansanshi Mining plc, a subsidiary of First Quantum Minerals. The remaining 20% is owned by the state through the parastatal Zambia Consolidated Copper Mines Ltd (ZCCM). The mine is located approximately 10 km north of the town of Solwezi and 180 km to the northwest of the Copperbelt town of Chingola. The mine has been in commercial operation since 2005.
Prior to June 2009, Kansanshi mine treated only two ore types: oxide and sulphide. All transitional ores mined during the period leading up to 2009 was stockpiled. The treatment of the minerologically complex transitional ores through a third train commenced in June 2009, but entailed numerous metallurgical challenges. This transitional ore (called 'mixed ore') could not previously be treated economically due to the high content of high acid-consuming (GAC) minerals. This made it unsuitable for the oxide float-leach circuit, and poor flotation response ruled out the conventional sulphide float circuit. The novel circuit involved a conventional sulphide pre-float followed by flotation involving staged-controlled potential sulphidization (CPS).
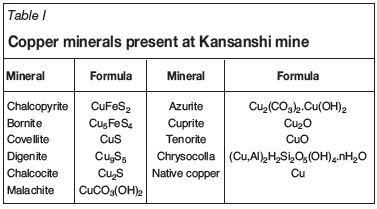
All the copper minerals constituting the alteration sequence from primary sulphides to carbonates or silicates are present in various proportions in the Kansanshi transitional ores (Table I). The alteration of primary sulphides starts with an impoverishment in iron to form covellite. Further oxidation results in an impoverishment in sulphur and enrichment in copper to form digenite, then chalcocite (Dunn and Muzenda, 2001). The final steps of alteration, leading to the formation of copper oxide minerals such as malachite or chrysocolla (a silicate) depend mainly on the composition of the gangue or fluid. The iron liberated during the alteration sequence can remobilize to form goethite or limonite. These iron oxides and hydroxides are particularly prejudicial for flotation if they precipitate on the surface of the copper minerals because they are not collectable with xanthates (Woods, 2003).
The traditional method applied for the flotation of copper oxide or mixed ores is sulphidization, which was first developed with industrial success on Pb-Zn oxide ores in Australia (Crozier, 1991). The method involves multistage addition of sodium sulphide (Na2S), sodium hydrogen sulphide (NaHS), or ammonium sulphide (NH4)2S as a sulphidizing agent, together with xanthate collectors such as potassium amyl xanthate (PAX) (Kongolo et al., 2003; Mwema and Mpoyo, 2001). The effectiveness of sulphidization for flotation of oxidized sulphides has also been demonstrated (Newell et al., 2006). When introduced in the slurry, the sulphidizer dissociates into the species H2S, HS- or S2-, depending on the pH. These ions react with the copper oxide minerals to form a sulphide layer on the surface of the oxide minerals (Zhou and Chander, 1992). The sulphidizer concentration can be controlled by measuring the ESpotential of the pulp using a sulphide ion-selective electrode. The reaction between the sulphidizer and malachite is optimum at a potential of -500 mV (Jones and Woodcock, 1978). However, xanthate flotation is depressed at this potential. Oxidation of sulphide ions in excess is then necessary to reach the optimal potential of -300 mV (Ferron and Manu, 1994). This leads to the formation of reducing agents such as thiosulphates, which are not necessarily indifferent in the flotation process (Castro et al., 1974; Soto and Laskowski, 1973).
Direct collectors, such as fatty acids and hydroxamates, have also been developed for the flotation of oxide minerals (Lee et al., 1998, 2009). The fatty acids have the drawback of being unselective over the carbonated gangue minerals and are therefore unsuitable for the Kansanshi mixed ores. Paquot et al. (2009) demonstrated the advantage of the sulphidization route over direct hydroxamates flotation.
The original Kansanshi mixed circuit configuration had six 150 m3 flotation cells. With this configuration, the first pair of cells was designated as a sulphide pre-flotation stage (referred to as a rougher stage). Sequentially, two sulphidization stages, each comprising two 150 m3 flotation cells, immediately followed the rougher stage. These were referred to as CPS 1 and 2. Two series of sulphidization conditioning tanks, to control sulphidizer addition against an Es/Eh potential and collector, preceded each sulphidization stage. The sulphidizer used was sodium hydrogen sulphide (NaHS).
Foremost among the metallurgical challenges with the mixed float was the loss of a certain class of copper minerals to the tails, despite indications that these minerals were fully liberated and floatable (Table II.). Paquot et al. (2009) and Ngulube et al. (2011) conducted several investigations to investigate this copper loss to tails, collecting samples across the whole circuit to assess liberation and mineral classes. Hundreds of laboratory flotation tests were also simultaneously conducted on flotation feed and final tails, under various plant conditions, in order to compare the performance of the plant with the ideal conditions in the laboratory.
The salient points from these studies were as follows.
During the sulphide pre-flotation (roughers), recoveries were generally predominantly in the particle size range -150 μm to +38 μm and, despite the fairly good mineral liberation, recovery was poor for the coarse (+150 μηι) and fines (-38 μηι) fractions. In the acid-insoluble copper range (sulphide mineralization), chalcocite and chalcopyrite showed the best flotation response (64 to 65% recovery) while covellite had the worst response among both primary and secondary sulphides. However, 'porous' chalcocite also had poor recoveries, mainly attributable to higher the amount of reagents required to activate surfaces that hardly contribute to hydrophobicity. Less than half of the liberated chalcopyrite (45%) was recovered in the rougher stage, mostly due to partial transformation of chalcopyrite to iron oxyhydroxides. These oxyhydroxide rims decreased the floatability of normally floatable mineral species. Even after sulphidization, liberated chalcopyrite and covellite were the sulphide species that displayed lower recovery
In the first sulphidization stage (CPS 1), the best recoveries were observed in the size range -150 μm to +75 μm. There was, however, very low recovery of the fines fraction (-38 μm). Compared with the roughers, there was better flotation response in CPS 1 with regard to sulphide recovery compared with the oxides. This was attributed mainly to the sulphidizer (NaHS) activating the tarnished or rimmed sulphide components. Secondary sulphides also showed a superior response to sulphidization
Malachite recovery started to become significant only in the second sulphidizing stage (CPS 2). Additionally, the coarse fraction sulphides were activated and recovered at this stage. However, only 50% of the liberated malachite was recovered. Malachite associated with iron oxides and hydroxides was not recovered
Major losses of the coarse but prior-activated particles in the CPS stages occurred in the cleaning circuit. However, chalcopyrite recovery was better than that of other secondary sulphides, malachite, and oxidized native copper in the cleaning section
The main copper losses in the final tails were attributed to liberated chalcopyrite and malachite (16% and 28%, respectively) that should be recovered if properly sulphidized. Malachite associated with iron oxides and hydroxides also contributed to a significant proportion of losses (20%). These losses are attributed mainly to poor sulphidization efficiency. Even lower recoveries were noticed for malachite stained by iron oxyhydroxides. Chalcopyrite had slow-floating tendencies as well. With the exception of covellite, the remainder of secondary sulphides had the best flotation responses. There was no evidence of chrysocolla floating
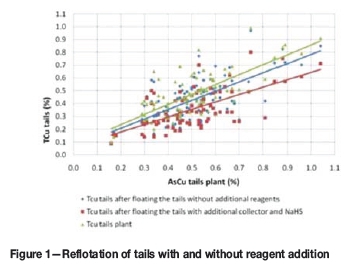
The average recovery difference between the laboratory experiments performed on the flotation feed and the plant results was around 9%. When floating the flotation tails in the laboratory without any additional reagents, the recovery was seen to improve by an average of 5%, while floating the tails with additional reagents resulted in improvements of up to 10% (Figure 1)
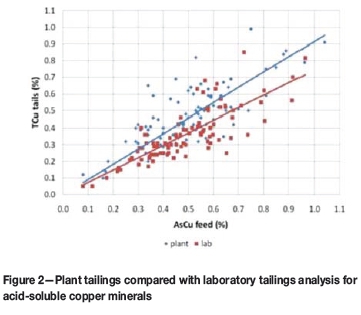
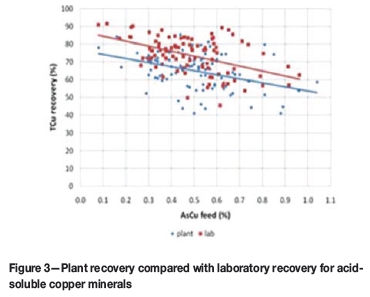
The higher the acid-soluble copper feed grade, the higher the observed improvement (Figure 2). The additional recovery was only around 2% when the acid-soluble copper feed grade was lower than 0.2%, but was as high as 15% when the acid-soluble copper feed grade was higher than 0.6% (Figure 3).
In this paper, we review the subsequent circuit changes and modifications that resulted from the findings of the work undertaken up to 2011, and the various challenges that these changes introduced. Relevant points from the above work were:
The need for increased residence time and stages for both the roughers and the CPS stages to capture more of the slow-activating mineral species and size fractions. Findings of the mineralogical studies, coupled with test work, pointed to insufficient residence time and less- than-efficient sulphidization. The slow-floating chalcopyrite needed more residence time and the fully liberated but lost malachite needed more sulphidization stages and residence time
The need to divert the mixed cleaner scavenger tails out of the mixed circuit because recirculating oxide minerals were increasing the possibility of this being lost to tails.
Plant expansions and results
Rougher/CPS expansions
The results of the plant and laboratory test work and mineralogy analysis were used to justify the extension of the mixed ore flotation plant, with the total changes costing approximately US$19 million. A more conservative recovery improvement of 5% was considered to justify this expenditure.
In mid-2011, six 300 m3 float cells were commissioned: four cells dedicated to the sulphide flotation circuit and the other two being flexible between mixed and sulphide ore flotation, depending on milling circuit configuration. The volume capacity of the mixed ore flotation plant increased from 900 m3 to 1500 m3 or 2100 m3, depending on whether the 300 m3 cells were used for mixed ore flotation. In late 2011, an extension of the CPS plant was commissioned to increase the number of sulphidization intervals to the current four stages. From an initial 900 m3 total capacity and two sulphidization stages, by the end of 2011 there was a total capacity of 2100 m3 and four sulphidization stages available. Subsequently, residence time increased from an average 20 minutes to between 30 and 60 minutes, depending on the milling circuit configurations.
Notably, during the same period in 2011, due to a shortfall of sulphide ore from the pit, it was decided that more of the mixed ores would be treated, utilizing the larger sulphide mills. This resulted in an 80% increase in daily throughput, effectively reducing the residence time through the mixed float circuit from 45 minutes to about 30 minutes.
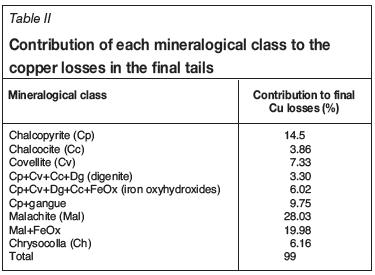
Figure 4 depict the flow sheets before and after the expansion. Figure 5 shows the effect of the additional float cells on the recovery from mixed ore.
Results from mixed circuit expansions
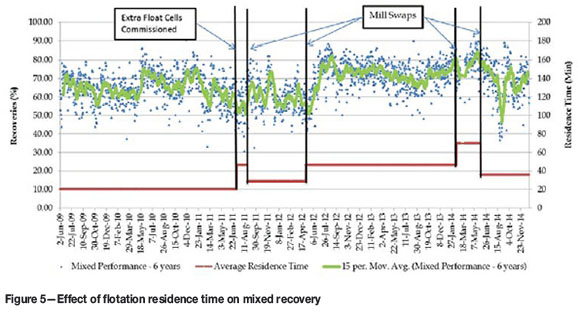
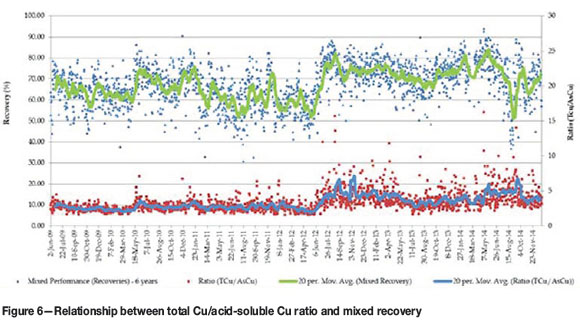
From the results obtained after the mixed circuit expansions, it could at first glance (Figure 5) be deduced that there was an immediate improvement, especially if the two periods of running the same mills on mixed ore (before June 2011 and after June 2012) are considered. However, as can be seen from Figure 6, recovery from the mixed ore through the Kansanshi circuit also has a very strong correlation with the total Cu/acid-soluble Cu ratio. The period 2011 and 2012 also coincided with the shift from treating predominantly stockpiled transitional ores to predominantly freshly mined ore from the pit. This ore was better grade-controlled for quality (acid-soluble Cu grade which was found to be the best approximation of the weathering profile). Initially, classification of mixed ores was based purely on the amount of acid-soluble Cu and GAC minerals in the material. Freshly mined material also showed less tarnishing. Figure 7 shows the progressive drop in sulphidizer consumption, especially during this same period of the circuit expansion.
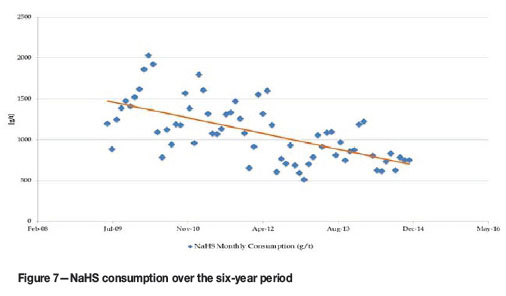
Clearly, a rigorous statistical approach was required to prove the benefits of the expanded circuit and the increased number of sulphidization stages.
The F-test result
As shown in Table III, two sets of recovery data were subjected to F-tests. The first set of data was for 192 days (192 data points) taken from on either side of the mill swap, which resulted in major changes in residence time. The new residence time was around 45 minutes on average, compared with 20 minutes before the extension. A recovery difference of 7.45% (higher at a residence time of 45 minutes) was noted with 100% confidence limit. Similarly, 204 data points were picked for before and after the mill swap in 2011/2012. This data-set showed a 10% recovery drop at a residence time of 30 minures, again with 100% confidence limit.
Cleaner/recleaner flotation
The increase in rougher and CPS stages on the mixed circuit was therefore successful in reducing copper losses. However, the resulting multiple sub-concentrate discharge points caused a drop in final product (final concentrate) quality. The higher mass pulls, and consequently increased volume reporting to the limited cleaner/recleaner flotation bank of only 288 m3 total volume, subdivided into nine float cells of 32 m3 each, led to higher insoluble components (silicates, aluminates, etc.) in the final combined concentrates. This resulted in significant smelter penalties. Figure 8 shows the trend of insolubles in the mixed ore final product, the recleaner concentrate. In August 2014, the cleaner/recleaner capacity was increased to ameliorate this problem, to great effect (Figure 8). Volumetrically, the capacity increased from a restricted 288 m3 to a substantial 1220 m3, consisting of three banks: 6 χ 150 m3 cleaners, 8 χ 30 m3 recleaners, and a further 5 x 16 m3 re-recleaners.
Comparative recovery models
The variation in mineralogy and TCu/AsCu contents in the feed causes significant fluctuations in recovery (Table IV). Flotation recovery from mixed ore generally varies between 50-90%. The NaHS consumption varies between 300 g/t and 3000 g/t. Robust multilinear regressions using iteratively reweighted least squares was used for modelling the plant flotation final tails and NaHS consumption. Performance of the plant is compared with the model on a daily basis. This also serves to highlight the variability in the results and reagent consumption. The correlation coefficients between the total Cu tails grade or NaHS dosage and the total Cu feed grade and acid-soluble Cu feed grade are given in Table V.
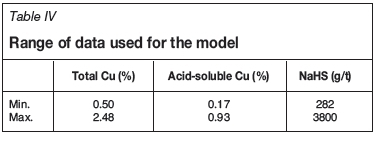
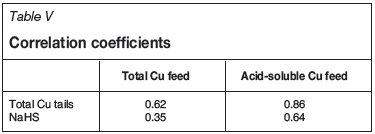
The equations are of the form:
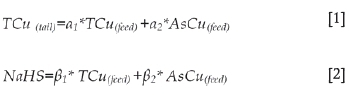
Figure 9 shows the models obtained for the total Cu grade of the tails, the corresponding recovery, and the model obtained for the NaHS consumption. Table VI gives the correlation coefficients between the models and the actual plant daily results.
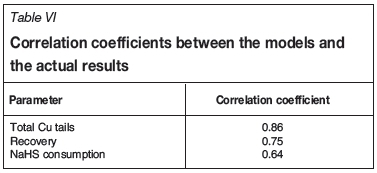
The main parameter affecting the total Cu recovery or the total Cu of the tails is the acid-soluble Cu feed grade.
Because the oxide minerals are more difficult to recover than the sulphides, a lower recovery (higher tails grade) can be expected when the acid-soluble Cu feed grade increases. It is thus not possible to set a fixed recovery target for the mixed ore flotation. However it is possible to set a tails grade target depending on the acid-soluble Cu feed grade. As a general rule, if the total Cu tails grade is lower than the acid-soluble feed grade, an average plant performance is achieved. The NaHS consumption depends mainly on the acid-soluble Cu feed grade. The fit for the model of the NaHS consumption is poor because it is calculated based on the variations in stock inventory levels. The model of the total Cu tails grade is not much different from the one obtained with the daily results, but the model of the NaHS consumption is very different, and the fit is much better; however, the application range has been reduced. Because the NaHS consumption is very variable, the stocks must be controlled carefully to avoid running out of reagents and to avoid holding excessive stock that would degrade. The model of the NaHS consumption can be used for monitoring the NaHS stocks, depending on the forecast for feed grades coming from the mine.
Conclusions
The envisaged recovery improvements from plant extensions and increasing the stagewise addition of sulphidizer were achieved. The increased residence time increased the recovery of slow-floating (tarnished) chalcopyrite. The probability of recovery of previously fully liberated but unrecovered malachite was increased by the addition of a further two stages of sulphidization at the tailings end of the old circuit. The mineralogical results showed that most chalcopyrite was recoverable only after being activated by sulphidizer in the first stage of sulphidization. Similarly, malachite recovery was achieved only in the second stage of sulphidization. Another gain in increasing the number of sulphidization stages is the recovery of liberated coarse-grained minerals.
References
Castro, S., Soto, H., Goldfarb, J., and Laskowski, J. 1973. Sulphidizing reactions in the flotation of oxidized copper mineral II. Role of the adsorption and oxidation of sodium sulphide in the flotation of chrysocolla and malachite. International Journal of Mineral Processing, vol. 1. pp. 151-161. [ Links ]
Crozier, R.D. 1991. Flotation, Theory, Reagents and Ore Testing. Pergamon Press. [ Links ]
Dunn, J.G. and Muzenda, C. 2001. Thermal oxidation of covellite (CuS). Thermochimica Acta, vol. 369, no. 1-2. pp. 117-123. [ Links ]
Ferron, C.J. and Manu, N.N. 1994. Recovery of copper oxide minerals by sulfidization flotation. Lakefield Research, Canada. 11 pp. [ Links ]
Lee, J.S., Nagaraj, D.R., and Coe, J.E. 1998. Practical aspects of oxide copper recovery with alkyl hydroxamates. Minerals Engineering, vol. 11, no. 10. pp. 929-939. [ Links ]
Lee, K., Archibald, D., Mclean, J., and Reuter, M.A. 2009. Flotation of mixed ore copper and sulphide minerals with xanthate and hydroxamate collectors. Minerals Engineering, vol. 22. pp. 395-401. [ Links ]
Jones, M.H. and Woodcock, J.T. 1978. Optimization and control of laboratory sulphidization of oxidized copper ores with an ion selective electrode. Proceedings of the Australasian Institute of Mining and Metallurgy, vol. 266. pp. 11-17. [ Links ]
Kongolo, K., Kipoka, M., Minanga, K. and Mpoyo, M. 2003. Improving efficiency of oxide-cobalt ores flotation by combination of sulphidisers. Minerals Engineering, vol. 16. pp. 1023-1026. [ Links ]
Mwena, M.D. and Mpoyo, M. 2001. Improvements of cobalt recovery in flotation of curo-cobaltiferous ore at Gecamines. Proceedings of Copper Cobalt Nickel and Zinc Recovery, Victoria Falls, Zimbabwe, 16-18 July 2001. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 1-9. [ Links ]
Paquot, F.X. and Ngulube, C. 2012. Development and optimization of mixed sulphide/oxide copper ore treatment at Kansanshi. Journal of the Southern African Institute of Mining and Metallurgy, vol. 115, no. 12. pp. 1253-1258. http://dx.doi.org/10.17159/24119717/2015/v115n12a15 [ Links ]
Paquot, F.X., Bastin, D., Mukutuma, A., and Delaney, A. 2009. Metallurgical performances of the sulphidization route and the direct alkyl hydrox- amates flotation of mixed carbonated copper-gold ores of the Kansanshi deposit. Proceedings of Flotation 2009, Cape Town, South Africa, 9-12 November 2009. Minerals Engineering International, Falmouth, UK. [ Links ] ♦
This paper was first presented at the, Copper Cobalt Africa Conference, 6-8 July 2015, Avani Victoria Falls Hotel, Victoria Falls, Livingstone, Zambia.
1 Paquot, F.X. and Ngulube, C. 2012. Development and optimization of mixed sulphide/oxide copper ore treatment at Kansanshi Journal of the Southern African Institute of Mining and Metallurgy, vol. 115, no. 12.