Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.6 Johannesburg Jun. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n6a5
PAPERS - COOPER COBALT AFRICA CONFERENCE
Optimization of circuits for pressure leaching of sulphide ores and concentrates
F. Saloojee; F.K. Crundwell
CM Solutions, Johannesburg, South Africa
SYNOPSIS
Pressure leaching is an option for copper recovery from chalcopyrite. Leaching takes place at high temperatures and pressures in the presence of an oxidizing agent. This work shows how the performance of pressure leaching circuits may be improved by optimizing the configuration of autoclaves and the heat removal method. The objective was achieved by creating an autoclave model that combines mass balances, energy balances, and population balances. A base case circuit consisting of a single autoclave was chosen, and this base case was compared with options for increasing capacity by adding more autoclaves to the circuit. These capacity increase options include circuits in which the additional autoclave is added in parallel, in series, and in series with thickening between the two autoclaves. The copper extraction, productivity, and cooling requirements for these options are compared. The series circuit with interstage thickening has the highest extraction and productivity; however, the cooling duty in the first autoclave is high. In addition to comparing circuit options, three options for heat removal were investigated: cooling coils, quench water, and flash recycling. The flash recycle option results in the highest copper extraction, and the quench water option the lowest copper extraction.
Keywords: pressure leaching, autoclave, flash recycling, heat removal
Introduction
Background
Copper sulphide ores and concentrates containing chalcopyrite are typically hard to leach under atmospheric conditions. Smelting of these ores may also be a challenge, due to the inability of smelting processes to handle low-grade ores, as well as impurities such as arsenic and antimony. Alternative hydrometal-lurgical routes to treating chalcopyrite are therefore required. One option is pressure leaching, which is the focus of this work.
In the pressure leaching process, sulphide ores and concentrates are leached in autoclaves at temperatures of 200 to 220°C and a pressure of about 3000 kPa (Schlesinger et al., 2011). An oxidizing agent, usually oxygen, is required to oxidize sulphide ions to sulphate ions. The oxidation of sulphur to sulphate is exothermic; consequently, a method of heat removal is necessary in order to maintain the temperature in the autoclave.
The dissolution of chalcopyrite is given by Equation [1]. Chalcopyrite in flotation concentrates is frequently accompanied by pyrite. Pyrite dissolution is given by Equation [2] (McDonald and Muir, 2007).
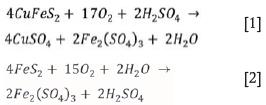
At temperatures above 200°C, the ferric sulphate reacts to form haematite, as shown in Equation [3]:
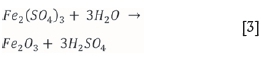
The kinetics of the leaching reactions can be described by the shrinking particle model. The reaction kinetics can be incorporated into a mathematical model of a continuous reactor, as described by Crundwell (1995). This approach is useful in the design of autoclave circuits, and has been used to design a copper leach autoclave at a base metals refinery (Crundwell, 2005), to predict the performance of a zinc pressure leaching operation (Crundwell and Bryson, 1992), and to model and optimize bacterial leaching reactors (Crundwell, 2000).
Aims and objectives
The aim of this work is to demonstrate two ways of optimizing a pressure leaching circuit, namely:
Optimizing the configuration of autoclaves
Optimizing the heat removal method.
These optimization studies were conducted by building a detailed model of the pressure leaching autoclave, including the reaction kinetics and heat removal methods. This model was then extended to simulate different configurations for autoclave circuits. The different configurations and options were compared in terms of copper extraction, productivity, and energy balance.
Problem statement
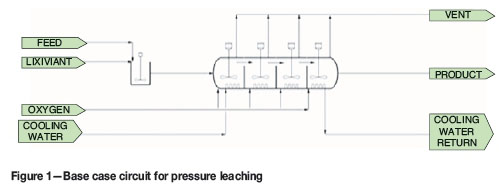
A hypothetical pressure leach plant has a single autoclave of volume V. Consider this as the base case circuit, shown in Figure 1. The temperature of the autoclave is maintained by running cooling water through coils in the vessel. The residence time in the autoclave is approximately one hour.
The plant is required to double its capacity. To maintain the residence time of the leach, an additional autoclave must be added to make up a total volume of 2 V. The autoclaves can be configured in one of the following ways:
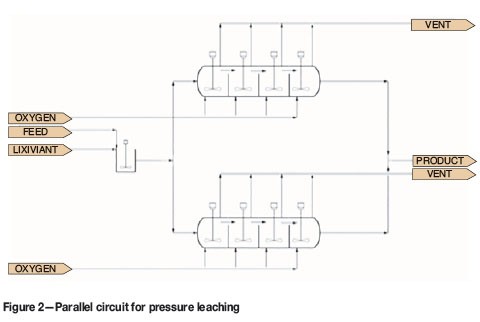
Two autoclaves, each with volume V, in parallel, as shown in Figure 2
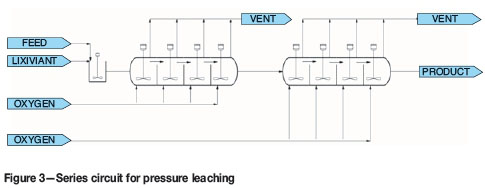
Two autoclaves, each with volume V, in series, as shown in Figure 3
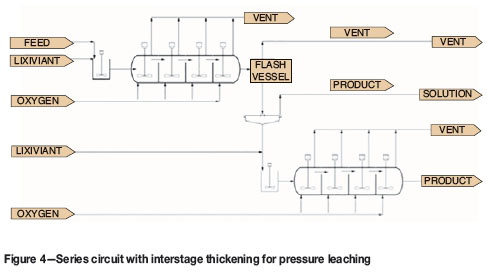
Two autoclaves, each with volume V, in series with a thickener between the two, as shown in Figure 4. The product from the first autoclave is thickened to 45% solids. The thickener underflow is then repulped with fresh lixiviant to make up a slurry with 30% solids.
The options for heat removal from the autoclaves are:
Cooling coils, as used in the base case circuit
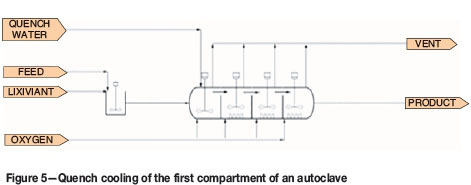
Quench cooling, shown in Figure 5, where cold water is added directly to the autoclave compartment
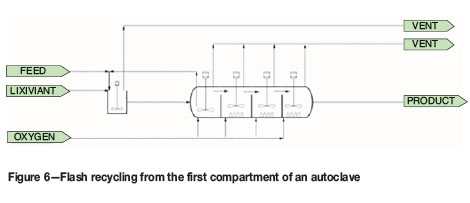
Flash recycling, shown in Figure 6. A portion of the slurry is removed from the first compartment of the autoclave and recycled to the feed tank. As the pressurized slurry enters the feed tank, water evaporates and the slurry temperature is reduced to the boiling point temperature. The proportion of recycled material is controlled to maintain the autoclave temperature.
The options for autoclave configuration and heat removal method are compared with the base case in terms of extraction and productivity.
Model framework
The autoclave model is a combination of mass balances, population balances, and energy balances. Each of these is discussed briefly in the sections below. Details of the model are available in the literature (Crundwell, 1994, 1995, 2000).
Inputs to the model include feed particle size, solids and solution throughput, reactor volume, retention time, reaction kinetics, gas mass transfer, and thermodynamic properties of the compounds. The mass balance, population balance, and energy balance equations are solved simultaneously in Cycad Process® software, resulting in product flows out of the reactor and cooling requirements.
Mass balance
The mass balance accounts for the conservation of mass. Mass balances for each component are included in the model. The mass balance equation for the solid and aqueous components is as follows:

where Q is the volumetric flow rate of the slurry feeding the reactor, Ciis the concentration of component i, r is the rate of formation of component i, and V is the volume of the slurry in the reactor.
A mass balance for dissolved oxygen accounts for gas mass transfer, according to Equation [5] (Crundwell, 2005):
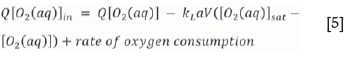
where kLa is the mass transfer coefficient and [O2(aq)]sat is the saturation concentration of oxygen at the autoclave temperature.
Population balance
A characteristic that distinguishes the modelling of leaching reactors from other reactors is the change in particle size as the reaction proceeds. The population balance accounts for the change in particle size of the solids as they react. This is important because the rate of dissolution is higher for smaller particles than for larger particles. The population balance is described by Rubisov and Papangelakis (1997) as a 'particle-counting' technique. The population balance equation is given in Equation [6]:
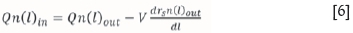
where ninand noutare the particle size density functions of the inlet and outlet, respectively, on a number basis, rsis the rate of shrinkage of particles in metres per second, and l is the particle diameter in metres.
The term Qn(l) nrepresents the material coming into the reactor with size l and the term Qn(l )out represents the material leaving the reactor with size l. The second term on the right-hand side represents the change in particle size due to reaction. This is made up of particles entering size class l due to shrinkage as well as particles leaving size class l dueto shrinkage. The term 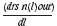 indicates that the reaction rate is dependent on particle size.
indicates that the reaction rate is dependent on particle size.
For a solid component i, the change in particle size distribution through the reactor represents the conversion of the component, as shown in Equation [7]:

Energy balance
The energy balance takes into account the heating or cooling requirements to maintain the reactor at the desired reaction temperature. The energy balance equation is:
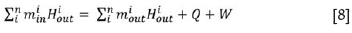
where minand moutare the masses of each component in the system entering and exiting the reactor, respectively, Hiinis the specific enthalpy of component i at the feed temperature, Hioutis the specific enthalpy of component i at the exit temperature, n is the number of components in the system, Q is the heat addition to the system, and W is the shaft work done. For comparison purposes in this model, the term for shaft work, W, is not considered.
The value of H¡ at temperature T is obtained from Equation [9]:

where Hi0 is the enthalpy of formation at a reference temperature Tref, and Cp is the heat capacity of component i.
The energy balance is solved by assuming that the reactor is maintained at a constant temperature. The cooling duty (Q), quench water requirements, or the flash recycle rate are adjusted so that the desired temperature is reached.
Evaluating the autoclave performance - the leaching number
The performance of leaching reactors depends on three factors: residence time, leaching kinetics, and particle size distribution. These factors are combined in the leaching number, defined by Crundwell (2005), according to Equation [10]:

where rsis the rate of shrinkage of particles, - is the mean residence time, and l is the mean particle size.
The leaching number can be interpreted as follows: in order to improve the performance of the reactor, the value of the leaching number must be increased. This can be achieved in three ways: increasing the intrinsic leaching rate, increasing the residence time, and decreasing the particle size. The value of the intrinsic leaching rate can be increased by increasing the temperature or the concentrations of reagents, or by adding a catalyst.
Model parameters
Process description
Sulphide concentrate is repulped in water to make up a slurry. The slurry is pumped to the first compartment of an autoclave. The autoclave has four compartments. Slurry flows from one compartment to the next over weirs which separate the compartments. Oxygen is fed to each compartment via gas nozzles. Unreacted oxygen is vented from the top of the vessel.
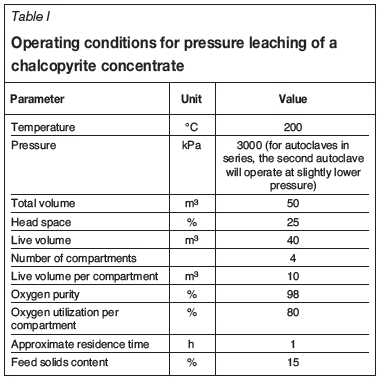
Operating conditions
The operating conditions of the autoclave are given in Table I.
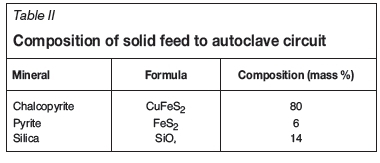
Feed composition
The feed to the process is a concentrate containing chalcopyrite and pyrite. The composition is the same as that of a concentrate investigated by McDonald and Muir (2007). For modelling purposes, the gangue mineral is assumed to be quartz. The feed composition is shown in Table II.
Feed particle size distribution (PSD)
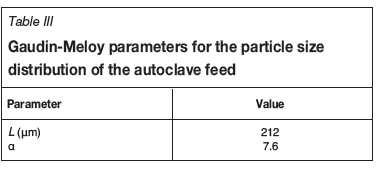
Particle size data was obtained for a chalcopyrite concentrate. The data was fitted to the Gaudin-Meloy model (Meloy and Gumtz, 1969), shown in Equation [11]:

where P(í) is the mass fraction of particles, L is the maximum particle diameter, and a is a value indicating the spread of the distribution. The fitted values for L and a are given in Table III.
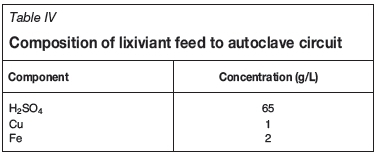
Lixiviant composition
The lixiviant for pressure leaching is usually made up of raffinate, with additional sulphuric acid to make up the desired concentration. The lixiviant composition used for the model is shown in Table IV. The lixiviant temperature is assumed to be 50°C.
Reaction kinetics
The dissolution of chalcopyrite and pyrite results in the formation of copper sulphate and ferric sulphate in solution. Pyrite dissolves to form ferrous sulphate in solution. It is assumed that these reactions follow the shrinking particle model with surface reaction control. The addition of oxygen results in the oxidation of ferrous iron to ferric iron. The ferric sulphate is hydrolysed to form haematite. The mass transfer of oxygen from the gas phase to the aqueous phase is also modelled as a reaction. Kinetic expressions for the dissolution and hydrolysis reactions were obtained from the literature (Langová and Matysek, 2010; McDonald and Muir, 2007; Papangelakis and Demopoulos, 1991; Vracar and Cerovic, 1997). The reactions and their rates are given in Table V.
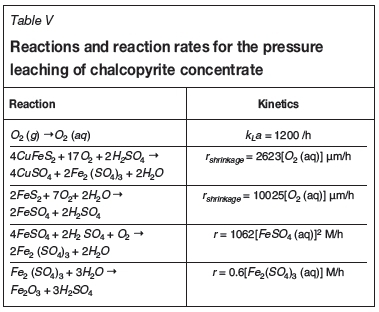
In addition to these reactions, evaporation of water also occurs. The amount of water evaporated is calculated by assuming that the off-gas is saturated with water vapour. The saturation concentration of the gas stream is calculated from the temperature of the reactor.
Base case simulation results
Results from the simulation of the base case circuit are presented in this section
Particle size
The change in particle size of chalcopyrite down the reactor is shown in Figure 7 as a density function. Pyrite follows a similar trend.
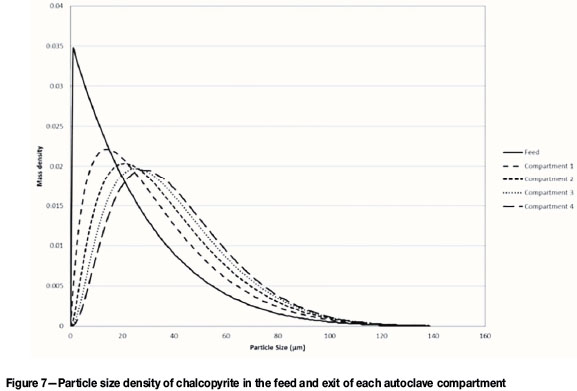
According to Figure 7, the mean particle size increases down the autoclave. This is counter-intuitive since particles become smaller as they react. This phenomenon has been explained by Crundwell et al. (2013) as follows: If the spread of the feed PSD is narrow, the mean particle size decreases as the reaction proceeds, as expected. However, if the spread of the feed PSD is wide, the mean particle size will increase. The smaller particles react completely and thus no longer contribute to the PSD. The mean size therefore moves closer to the size of the larger particles.
The spread of the feed PSD can be quantified by the covariance, given in Equation [12]:

where a is the standard deviation of the particle size and μ is the mean particle size.
According to Crundwell et al. (2013), covariance values less than 0.5 result in a decrease in mean particle size, while values greater than 0.5 result in an increase.
Copper and sulphur extraction
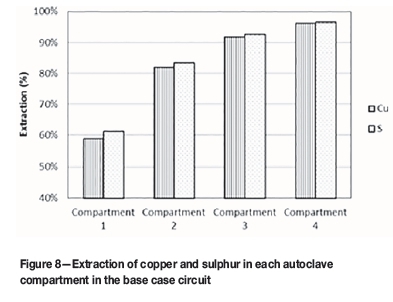
The extraction of copper and sulphur in each compartment of the autoclave for the base case is shown in Figure 8. The overall copper extraction for the base case is 96.15%.
Energy balance
The energy requirements for cooling each compartment of the autoclave are shown in Table VI. The highest degree of sulphur oxidation occurs in the first compartment. However, the cooling duty is lower than that of the second compartment because the feed to the first compartment is at a temperature of 49°C. In the autoclave, this stream is heated to the operating temperature of 200°C, which results in a decrease in cooling requirements.
Effect of gas mass transfer
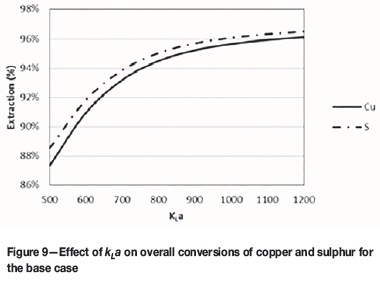
The effect of mass transfer of oxygen from the gas phase to the aqueous phase was investigated by varying the mass transfer coefficient and recording the resulting extractions of copper and sulphur. The same mass transfer coefficient was used in each compartment of the autoclave. The results are shown in Figure 9. Results show that higher values of kLa result in higher extractions of copper and sulphur.
Comparison of different autoclave configurations
In this section, the different circuit configurations are compared in terms of extraction, productivity, and energy requirements.
Copper and sulphur extraction
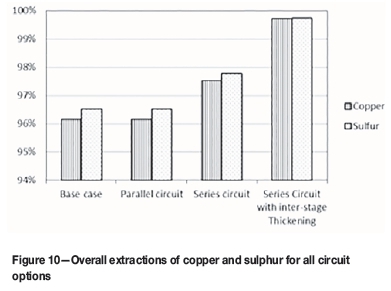
The overall extractions of copper and sulphur are shown in Figure 10. The base case and parallel circuits yield the same values for extraction. This is expected. The series circuits give slightly higher extractions of copper and sulphur. The highest extraction is achieved in the series circuit with interstage thickening. This is because solution is removed after the first autoclave. A smaller volume of solution is added to make up a slurry with 30% solids. The residence time of the solids in the second autoclave is therefore significantly increased.
Productivity
The productivity of a reactor is a measure of the amount of material reacted per unit volume of the reactor, as given in Equation [13].
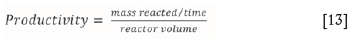
The productivity of sulphur leaching was measured. Results are shown in Table VII.
Energy balance
The cooling requirements for each compartment of the autoclave in the base case circuit were presented in Table VI. The cooling requirements for each autoclave compartment in the remaining circuits are presented in Table VIII.
Comparison of heat removal options
Copper extraction
The heat removal options are compared in terms of copper extraction down the autoclave. Results are shown in Figure 11. In the base case option, cooling coils are used. For the quench cooling option, copper conversion is lower than for the base case option. This is because the addition of quench water reduces the residence time in the autoclave.
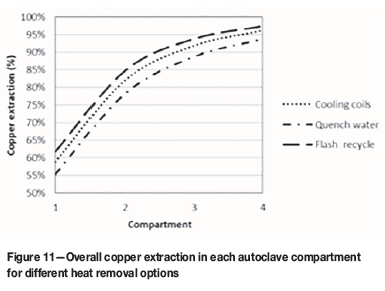
The highest extraction of copper is achieved with the flash recycle option. This is because water is removed from the recycled material by evaporation. The residence time of the solids in the first compartment is therefore increased.
Conclusions
An autoclave model has been created to simulate the pressure leaching of a material containing chalcopyrite and pyrite. The model was used to compare different autoclave configurations and heat removal options.
The options for configuration of a circuit with two autoclaves are parallel, series, and series with interstage thickening. These options were compared in terms of copper extraction, productivity, and energy balance. The series circuit has higher copper extraction and productivity than the parallel circuit. The series circuit with interstage thickening has the highest extraction and productivity. This is because the thickening step removes solution and thus increases the residence time of solids in the second autoclave.
The downside of the two series circuits is the high cooling requirement in the first autoclave. The first compartment requires heat addition because of the increased feed rate. However, the second compartment requires 7 MW of cooling, which may not be practical. These options need to be compared in terms of capital costs.
The cooling options are cooling coils, flash recycling, and quench cooling. These were compared in terms of copper extraction and exit copper concentration. The quench cooling option results in the lowest copper extraction and concentration. This is because the quench water reduces the residence time in the autoclave. The flash recycle option gives the highest copper extraction and productivity. The reason is that flashing of the material from the first compartment increases the residence time of the solids in that compartment.
References
Crundwell, F.K. 1994. Mathematical modelling of batch and continuous bacterial leaching. The Chemical Engineering Journal, vol. 54. pp. 207-220. [ Links ]
Crundwell, F.K. 1995. Progress in the mathematical modelling of leaching reactors. Hydrometallurgy, vol. 39. pp. 321-335. [ Links ]
Crundwell, F.K. 2000. Modeling, simulation and optimization of bacterial leaching reactors. Biotechnology and Bioengineering, vol. 71, no. 4. pp. 255-265. [ Links ]
Crundwell, F K. 2005. The leaching number: its definition and use in determining the performance of leaching reactors and autoclaves. Minerals Engineering, vol. 18. pp. 1315-1324. [ Links ]
Crundwell, F.K. and Bryson, A.W. 1992. The modelling of particulate leaching reactors - the population balance approach. Hydrometallurgy, vol. 29. pp. 275-295. [ Links ]
Crundwell, F.K., du Preez, N., and Lloyd, J.M. 2013. Dynamics of particle size distribution in continuous leaching reactors and autoclaves. Hydrometallurgy, vol. 133. pp. 44-50. [ Links ]
LangovA, S. and Matysek, D. 2010. Zinc recovery from steel-making wastes by acid pressure leaching and hematite precipitation. Hydrometallurgy, vol. 101. pp. 171-173. [ Links ]
Meloy, T.P. and Gumtz, G.D. 1969. The fracture of single, brittle, heterogeneous particles-statistical derivation of the mass distribution equation. Powder Technology, vol. 2, no. 4. pp. 207-214. [ Links ]
McDonald, R.G. and Muir, D.M. 2007. Pressure oxidation leaching of chalcopyrite 1. Comparison of high and low temperature reaction kinetics and products. Hydrometallurgy, vol. 86. pp. 191-205. [ Links ]
Papangelakis, V.G. and Demopoulos, G.P. 1991. Acid pressure oxidation of pyrite: reaction kinetics. Hydrometallurgy, vol. 26. pp. 309-325. [ Links ]
Rubisov, D.H. and Papangelakis, V.G. 1997. Solution techniques for population balance equations as applied to heterogeneous aqueous processes in stirred tank reactors. Computers in Chemical Engineering, vol. 21, no. 9. pp. 1031-1042. [ Links ]
Schlesinger, M.E., King, M.J., Sole, K.C., and Davenport, W.G. 2011. Extractive Metallurgy of Copper. 5th edn. Elsevier. [ Links ]
Vračar, R.Ž. and Cerović, K.P. 1997. Kinetics of oxidation of Fe(II) ions by gaseous oxygen at high temperatures in an autoclave. Hydrometallurgy, vol. 44. pp. 113-124. [ Links ] ♦
This paper was first presented at the, Copper Cobalt Africa Conference, 6-8 July 2015, Avani Victoria Falls Hotel, Victoria Falls, Livingstone, Zambia.