Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.1 Johannesburg Jan. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n1a15
PAPERS - NUCLEAR MATERIALS DEVELOPMENT CONFERENCE
Insights into the potential for reduced refractory wear in silicomanganese smelters
J.D. SteenkampI; P.C. PistoriusII; J. MullerIII
IMintek, Randburg South Africa
IICarnegie Mellon University, Pittsburgh PA USA
IIIEXXARO Resources, Pretoria West, South Africa
SYNOPSIS
Excavation of an industrial-scale submerged arc furnace, utilized in the production of silicomanganese, identified two high-wear areas in the refractory lining: the tap-hole, built with cold rammed carbon paste and SiC bricks, and the hearth, built with cold rammed carbon paste. To obtain insight into the potential causes of wear, thermodynamic calculations were conducted on eight sets of data, obtained for seven furnaces on three plants based in South Africa. FACTsage software and associated databases - FACTPS, FToxid, and FSstel - as well as the Mn-Fe-Si-C database of Tang and Olsen (2006), were applied. Theoretical indications are that chemical reaction between carbon refractory and slag, as well as dissolution of carbon and SiC refractory in metal, contributed to tap-hole refractory wear; and dissolution of carbon in metal contributed to hearth refractory wear.
Keywords: tap-hole, silicomanganese, submerged arc furnace, refractory, refractory wear, FactStage
Introduction
During the excavation of a 48 MVA submerged arc furnace (SAF) used for silicomanganese (SiMn) production, it was found that the tap-hole and hearth were high refractory wear areas (Steenkamp, 2014). In both these high-wear areas, carbon-based cold ramming paste formed the hot face refractory lining (crucible). The wear profile of this SAF is presented in Figure 1.
The hot face refractory lining was formed by cold ramming of high-grade carbon ramming material. This material consisted of 50-70% anthracite, 15-25% graphite, 6-12% resin, 2-7% tar, 1-5% clay, and 1-5% alumina. The tap-hole was built using SiC bricks, consisting of 75% SiC, 23.4% Si3N4, 0.3% Fe2O3, 0.3% Al2O3, and 0.2% CaO.
In the tap-hole area, the wear of the SiC refractory material was estimated at 0.4 t, and that of the high-grade carbon ramming material, at 1.9 t (Steenkamp, Pistorius, and Tangstad, 2015). The amounts of slag and metal tapped during six years of operation of the tap-hole (September 2007 to April 2013) were estimated at 33 088 t (Steenkamp et al., 2015) and 41 360 t, respectively.
The original thickness of the high-grade carbon ramming paste installed in the hearth was 850 mm, of which a minimum of 600 mm was seen to remain after ten years of operation (April 2003 to April 2013). Over the lifetime of the hearth refractory, 384 000 t of SiMn metal was tapped from the furnace (Gous, 2015).
The difference in metal exposure of hearth and tap-hole is due to the fact that the furnace had two single-level tap-holes, and that the tap-holes were rebuilt during the lifetime of the hearth refractory. Further details of the refractory design, and history of operations have been discussed elsewhere (Steenkamp et al, 2014, 2015; Steenkamp, 2014).
Analysis of the potential for chemical wear in the tap-hole area of the SAF excavated in 2013 (Figure 1) was included in a larger study on tap-hole wear in SAFs producing SiMn (Steenkamp, 2014). In that study the potential for chemical reaction between slag (and metal) and refractory materials being responsible for wear in a single-level tap-hole where slag and metal are tapped typically at 1600°C was tested.
It was previously established that reaction between silicomanganese slag and carbon-based tap-hole refractory is possible (Steenkamp, 2014). Predictions by thermodynamic calculations were supported by laboratory-scale experiments with nominally pure materials as well as industrial materials (Steenkamp, 2014), as reaction products SiC and SiMn droplets formed. For the reaction of C-based refractory with slag, if the SiC reaction product were to form an in situ refractory layer at the slag/refractory interface, the potential for refractory wear by chemical reaction would be reduced (Lee and Moore, 1998). However, in all laboratory-scale experiments conducted, the SiC that formed detached from the surface of the carbon-based refractory (Steenkamp, 2014), as was found by others (Molnâs, 2011). Thermodynamic calculations on the industrial materials applied, and produced, in the SAF excavated in 2013 (Figure 1) supported the observation that reaction between silicomanganese slag, and carbon-based tap-hole refractory is possible. Mass transfer calculations (SiO2 only) indicated that chemical reaction would not be the only wear mechanism applicable.
The previous study (Steenkamp, 2014) focused primarily on refractory/slag interaction. The thermodynamic calculations in that study were based on published slag and metal compositions, as well as plant data for the SAF excavated in 2013, where the average metal and slag compositions were calculated for daily compositions over a 4-month period prior to excavation - Table I and Table II.
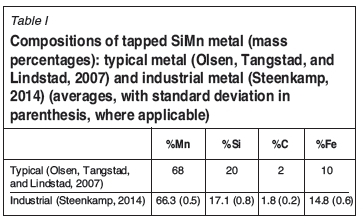
Based on these calculations, the slag can react with graphite at 1600°C to form solid SiC, liquid metal, and gas. MnO is reduced to form Mn in liquid metal and in gas; SiO2 is reduced to form solid SiC, Si dissolved in liquid metal, and SiO in the gas. Carbon would dissolve in the Mn-Si-C metal.
The potential for reaction of metal with carbon was assessed by using the FSstel database in FactSage 6.4 (Bale et al., 2002). It was found that the carbon solubility predicted with FactSage is very similar to that predicted by Tang and Olsen (2006), if slightly lower (see Figure 2). Most importantly for this discussion, the silicon content at double saturation of the metal (saturation with both graphite and silicon carbide) is essentially the same; see Table III. Metal containing more silicon than required for double saturation would tend to dissolve carbon and precipitate silicon carbide if it were to come into contact with carbon. At 1600°C, the silicon concentration at double saturation is approximately 16.3% (by mass); as Table I indicates, typical industrially produced silicomanganese contains more silicon than this, and hence would be expected to dissolve carbon-based refractory.
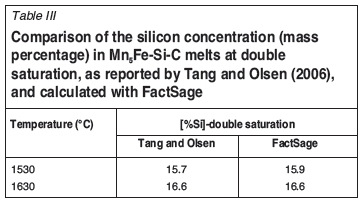
To test these observations (possibility of SiC formation through reduction of SiO2 in tapped slag; dissolution of C into tapped metal), the plant data, for which the averaged compositions are reported in Table I and Table II, was analysed in more detail. The results were presented at INFACON XIV (Steenkamp and Pistorius, 2015). An extract was included in the first part of the paper.
To test whether metal dissolving the carbon in the refractory would contribute to the wear observed in the hearth, the study was expanded to include tap data from seven furnaces (designated Furnace A to Furnace G), based at three different plants (designated Plant A to Plant C) in South Africa. The results are discussed in the second part of this paper.
Methodology
The first set of calculations was based on bulk chemical compositions of daily average slag and metal analyses, calculated from samples taken per tap. The tap dates are indicated in Table IV.
The second set of calculations was based on bulk chemical compositions of metal sampled per tap. Table V summarizes the tap dates and number of taps for each of the seven furnaces, based at the three different plants in South Africa.
The average slag and metal compositions, and standard deviations between analyses, as-received, were calculated per furnace, and are reported in Table VI.
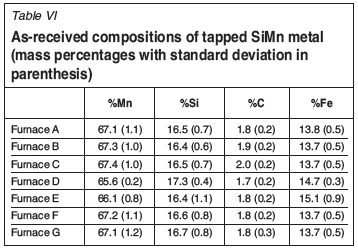
In preparation of the thermodynamic calculations, the received metal and slag analyses were normalized.
To correct the slag compositions for entrained metal, it was assumed that all Fe reported in the slag analyses was present as entrained metal droplets, and that these droplets had the same composition as the daily tapped bulk metal (containing mainly Fe, Si, Mn, and C). The assumption was previously validated by SEM-EDS analysis on an industrial slag sample obtained from the same plant (Steenkamp, Pistorius, and Tangstad, 2015). Mass balance calculations were conducted to correct the reported slag composition for FeO (recalculated to be zero), SiO2, and MnO (recalculated, based on entrained metal mass, to be lower). As part of this calculation, the mass of metal entrained per 100 g slag, was estimated.
The average slag and metal compositions, and standard deviations between analyses normalized, were calculated per furnace, and are reported in Table I, Table II, and Table VII.
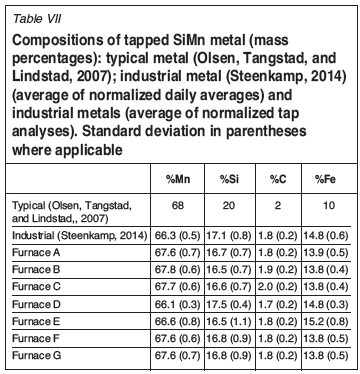
Thermodynamic calculations were conducted in FactSage 6.4, using the FToxid, FSstel, and FactPS databases, and the liquid Mn-Fe-Si-C database of Tang and Olsen (2006). Process temperatures between 1530°C and 1600°C are required to produce Mn7FeSiCsat containing 17% Si in equilibrium with slag where the SiO2 activity ranges between 0.2 and saturation, at 1 atmosphere pressure (Olsen, Tangstad, and Lindstad, 2007). Tapping temperatures are typically 50°C lower than process temperatures (Olsen, Tangstad, and Lindstad, 2007; Steenkamp, 2014). Therefore, calculations performed to study tap-hole refractory interaction with slag/metal were for 1550°C and 1600°C; and 1550°C, 1600°C, and 1650°C to study hearth refractory/metal interaction. In all instances a pressure of 1 atmosphere was specified. Selected reference states were graphite for carbon, solid SiC for SiC, and cristobalite for SiO2.
Results and discussion
Potential for metal dissolving refractory in tap-hole
To test whether the metal would tend to dissolve carbon or SiC, contributing to tap-hole refractory wear, the activities of carbon (graphite reference state) and silicon carbide (solid SiC as reference state) in the metal, were calculated for 1600°C and 1550°C. The calculations were mainly performed in two ways: first, the metal was taken to be fully liquid (allowing no precipitation of solids); in this case, activities of SiC and graphite could exceed unity. Second, precipitation of solids allowed (graphite or SiC, in this case) and changes in composition and saturation conditions were noted. In these calculations, the analysed concentrations of Si, Mn, Fe, and C were used.
To give a more general indication, a graphite/silicon carbide saturation diagram for 1600°C was also calculated for a Mn:Fe mass ratio of 4.47 (the average mass ratio of the daily average tapped metal compositions), using the FSstel database (see Figure 3). The double saturation point at 1600°C was found to be 2.04% C and 16.26% Si for the Si-Mn-Fe-C metal with a Mn:Fe mass ratio of 4.47. Superimposed onto the metal saturation diagram in Figure 3 are the daily average tapped metal compositions for the 4-month period.
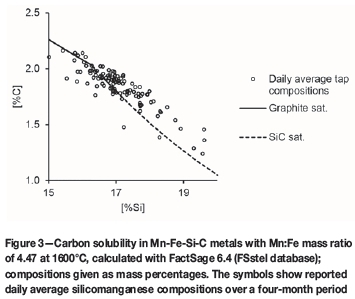
Figure 3 indicates that, as was the case for the average metal composition (Steenkamp, 2014), many of the metal compositions were supersaturated in SiC, and not saturated in C. To illustrate this differently, the calculated activities of C (with respect to graphite) and SiC for the case where precipitation of solids was suppressed are reported in Figure 4 (FSstel database). Figure 5 reports the calculated activities of C (with respect to graphite) and SiC for the case where precipitation of solids was allowed for both the FSstel and Tang and Olsen (2006) databases.
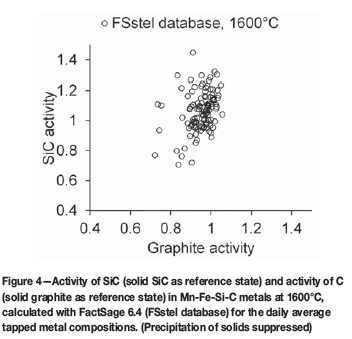
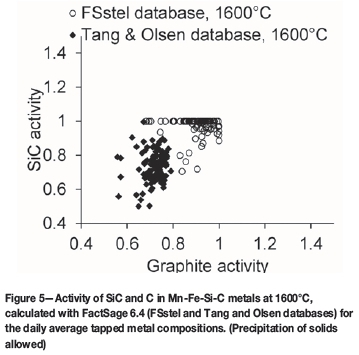
Based on the calculated activities of SiC and C (FSstel), for the case where precipitation of solids was suppressed (Figure 4), 67% of the metal taps had SiC activities greater than unity, whereas only 20% of the taps were supersaturated in graphite. In the cases where solid precipitation was allowed to proceed to equilibrium (Figure 5), the proportion of graphite-saturated melts was even smaller (Table VIII); this is because SiC precipitation (which would occur in SiC-saturated melts) would remove carbon from solution, moving the metal composition even further from graphite saturation. Where the calculations were based on the Tang and Olsen (2006) database, the calculated activities of SiC and C, were lower than for FSstel (Table VIII).
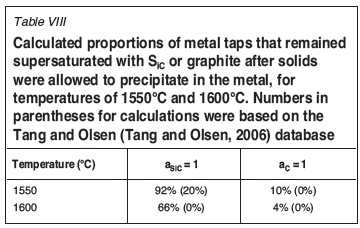
It is worth noting that the predicted proportion of graphite-saturated melts remained low, even at a lower temperature of 1550°C (Table VIII). This is mainly because the silicon content at double saturation does not change much upon cooling to the lower temperature (as also indicated by Figure 2). This means that lowering the metal temperature (or slight cooling of the tap-block) would not necessarily help to avoid chemical wear of carbon tap-blocks by silicomanganese. This contrasts with the potential for reaction of SiO2 in the slag with carbon (next section), which would be eliminated by cooling to 1550°C.
Potential for slag reduction refractory wear by slag in the tap-hole
The driving force for SiC formation, according to Equation [1], depends on the activity of SiO2 in the slag, assuming unit activity for all other components.

Should the activity of SiO2 in the slag be higher than the equilibrium activity (calculated to be 0.17 at 1600°C; reference state cristobalite), chemical attack of carbon refractory by SiO2 in the slag would be possible. Calculated SiO2 activities (for 1600°C) are reported in Figure 6. For all slag compositions, the activity of SiO2 was higher than the equilibrium activity of SiO2 for SiC formation with C at 1600°C, i.e. all slag compositions had the potential to react chemically with C-based refractory materials. However, a decrease in temperature to 1550°C would render SiO2 non-reactive towards carbon (Figure 6). (SiO2 activities in the slag do change with temperature; these activities were also recalculated for 1550°C, but were found to be only slightly lower from the 1600°C values, shown in Figure 6.)
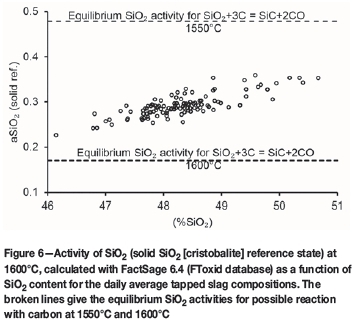
Also illustrated in Figure 6 is the effect of variation in slag composition on the activity of SiO2 in the slag for a constant SiO2 content (mass percentage), and the expected increase in SiO2 activity with increased SiO2 content of the slag.
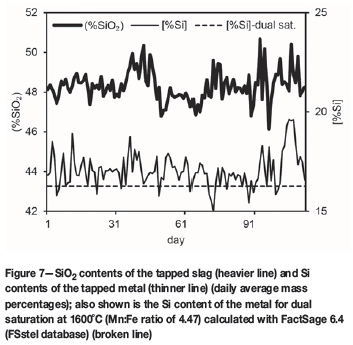
In Figure 7, the SiO2 contents of the tapped slag, and the Si contents of the tapped metal, are summarized (daily average; mass percentages) to illustrate variations in both. Also indicated in Figure 7 is the Si content of the metal for double saturation at 1600°C (Mn:Fe ratio of 4.47; broken line), emphasizing that most metal compositions have silicon concentrations greater than dual saturation.
The velocity and diffusion boundary layers that are taken into account in mass transfer calculations (Steenkamp, 2014) would develop near the wall inside a tap-hole, due to the effects of viscosity. Muller (2014) demonstrated that metal entrained in the slag phase has an influence on the effective viscosity, and therefore also on the volumetric flow rate through a tap-hole. Both factors would influence the rate at which SiO2 is transferred to the slag/refractory interface, where chemical reaction between slag and refractory would occur.
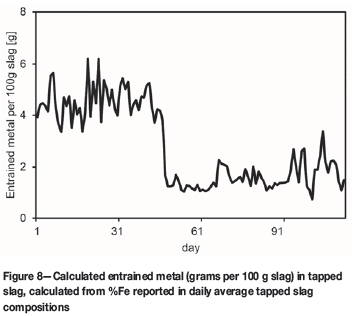
In Figure 8 the entrained metal concentration (grams per 100 g slag) in slag samples, calculated for the daily average tapped slag compositions, is presented. A distinct shift in entrainment is observed after 45 days of production (for unknown reasons).
Potential for metal dissolving refractory in hearth
To test whether the metal would tend to dissolve carbon and therefore contribute to hearth refractory wear, the activities of carbon (graphite reference state) and silicon carbide (solid SiC as reference state) in the metal were calculated for 1550°C, 1600°C, and 1650°C. SiC was included in the calculations, as it could potentially be utilized as an alternative hot face lining material in the hearth. In the calculations, precipitation of solids (graphite or SiC) was allowed, and changes in composition and saturation conditions were noted. In these calculations, the normalized concentrations of Si, Mn, Fe, and C were used.
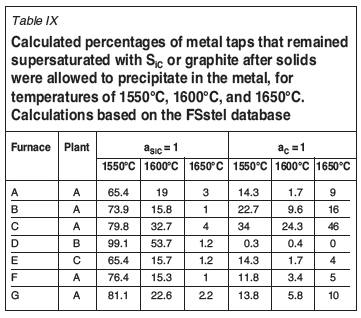
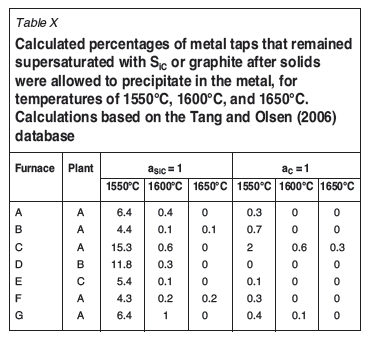
Based on the calculated activities of SiC, and C (FSstel, Table IX), the number of metal taps that had SiC activities greater than or equal to unity decreased with increasing temperature. This was not the case for supersaturation in graphite: from 1550°C to 1600°C the number of taps decreased, but from 1600°C to 1650°C the number of taps increased. Where the calculations were based on the Tang and Olsen (2006) database (Table X), the number of supersaturated metal taps decreased with increase in temperature, both from a graphite and a SiC saturation perspective. Again where the calculations were based on the Tang and Olsen (2006) database (Table X), the calculated activities of SiC and C, were lower than for FSstel (Table IX). This was the case for all furnaces from all plants.
The results are presented differently in Figure 9 (data from FSstel) and Figure 10 (data from Tang and Olsen): the cumulative fraction of metal taps, as a function of SiC or graphite activity (after solids were allowed to precipitate in the metal), for temperatures of 1550°C, 1600°C, and 1650°C, per furnace. The differences in results obtained from the two different databases - discussed before - were again observed.
If the deviation of aC or aSiC from unity is taken as an indication of the driving force for refractory wear through dissolution into the metal, the driving force for SiC dissolution at 1550°C is lower than for carbon. At 1650°C the opposite seems to occur, with the driving force for SiC dissolution being slightly higher than for carbon. The effect on refractory wear is illustrated in Figure 11, where SiC solubility has a greater sensitivity to increases in temperature than carbon.
As was done for the thermodynamic calculations studying slag/refractory interaction (Steenkamp, 2014), the next step would be to test these observations on laboratory scale.
Conclusions
During excavation of a 48 MVA submerged arc furnace used for SiMn production, two high-wear areas were found: the tap-hole area and the furnace hearth. Carbon-based refractory material formed the hot face refractory. Analysis of daily average tapped slag and metal compositions produced in the four months prior to excavation confirmed the potential for refractory wear in the tap-hole through C dissolution in the metal and SiC formation by chemical reaction with SiO2 in the slag. Similar analysis of tapped metal data from seven SiMn smelters situated at three plants in South Africa indicated the potential for refractory wear in the hearth through C dissolution in the metal. From a metal dissolution perspective, theoretical indications are that SiC would be more suitable as tap-hole refractory, and C as hearth refractory. Experimental confirmation of the observations from thermodynamic calculations would be useful.
Acknowledgements
This paper is published with the permission of Mintek, after being reviewed internally by Isabel Geldenhuys, Glen Denton, and Herman Lagendijk. The authors would like to thank Oleg Ostrovski, and Merete Tangstad, for discussions around activity of C and SiC in metal, and SiO2 in slag produced in SiMn smelters, Kai Tang for the supply of the database, and management at the three different plants for supply of plant data.
References
Bale, C., Chartrand, P., Degterov, S., and Eriksson, G. 2002. FactSage thermochemical software and databases. Calphad, vol. 26. pp. 189-228. http://wiww.sciencedirect.com/science/article/pii/S0364591602000354 [ Links ]
Gous, J. 2015. Personal communication. [ Links ]
Lee, W.E. and Moore, R.E. 1998. Evolution of in situ refractories in the 20th century. Journal of the American Ceramic Society, vol. 81, no. 6. pp. 1385-1410. [ Links ]
MolnAs, H. 2011. Compatibility Study of Carbon-Based Refractory Materials utilized in Silicomanganese Production Furnaces. Norwegian university of Science and Technology. [ Links ]
Muller, J. 2014. Evaluation of HCFeMn and SiMn Slag Tapping Flow Behaviour Using Physicochemical Property Modelling and Analytical Flow Modelling. MSc thesis, University of Pretoria. [ Links ]
Olsen, S.E., Tangstad, M., and Lindstad, T. 2007. Production of Manganese Ferrometals. Tapir Academic Press, Trondheim, Norway. [ Links ]
Steenkamp, J.D. 2014. Chemical Wear of Carbon-based Refractory Materials in a Silicomanganese Furnace Tap-hole. PhD thesis, University of Pretoria. [ Links ]
Steenkamp, J.D., Gous, J.P., Pistorius, P.C., Tangstad, M., and Zietsman, J.H. 2014. Wear analysis of a taphole from a SiMn production furnace. Furnace Tapping Conference 2014, Muldersdrift, South Africa, 27-28 May 2014. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 51-64. [ Links ]
Steenkamp, J.D. and Pistorius, P.C. 2015. Chemical wear of carbon-based refractory materials in a silicomanganese furnace tap-hole. INFACON XIV, Kiev, Ukraine. pp. 505-510. [ Links ]
Steenkamp, J.D., Pistorius, P.C., and Tangstad, M. 2015. Chemical wear analysis of a tap-hole on a SiMn production furnace. Journal of the Southern African Institute of Mining and Metallurgy, vol. 115, no. 3. pp. 199-208. [ Links ]
Tang, K. and Olsen, S.E. 2006. Computer simulation of equilibrium relations in manganese ferrometal production. Metallurgical and Materials Transactions B, vol. 37. pp. 599-606. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2016. ISSN2225-6253. Parts of this paper was first presented at, INFACON XIV, Kyiv, Ukraine.














