Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.116 n.1 Johannesburg Jan. 2016
http://dx.doi.org/10.17159/2411-9717/2016/v116n1a14
PAPERS - NUCLEAR MATERIALS DEVELOPMENT CONFERENCE
Solvent extraction and separation of hafnium from zirconium using Ionquest 801
L. de Beer; D.J. van der Westhuizen; H.M. Krieg
Chemical Resource Beneficiation, North-West University, Potchefstroom, South Africa
SYNOPSIS
The solvent extraction (SX) characteristics of Ionquest 801 were investigated to determine if the selective extraction of hafnium (Hf) over zirconium (Zr) is possible. Firstly, Ionquest 801 was screened for its suitability as a function of acid concentration. The solvent consisted of 5wt % extractant, corresponding to a extractant to metal ratio (E:MZr) of 14:1, in cyclohexane with 5% v/v 1-octanol added as modifier. Aqueous solutions of ZrCl4 with 3 wt% HfCl4 were prepared by dissolution of the salts in HNO3 to obtain 1.0 g/l Zr(Hf)Cl4(aq) feed solutions (aged 24 hours). Subsequently, the extractant concentration was varied at optimal acid molarities. The most favourable extraction percentages were attained at 0.7 M HNO3 and an E:MZr ratio of 5:1. Artifices in the form of aqueous additives were used to maximize the Hf selectivity at these optimal conditions. These included the addition of 1.5 M NaCl, NaNO3, Na2SO4, and NaClO4.H2O, as well as tartaric, oxalic, mandelic, and citric acid with and without the addition of 6 wt% hydrogen peroxide (H2O2). Exploratory studies on the influence and usability of the most promising factors such as [SO-42], [H2O2], and combinations thereof in a SX system were undertaken.
Keywords: solvent extraction, Ionquest 801, PC88A, organophosphorus, hafnium, zirconium
Introduction
While zirconium (Zr) is used in a variety of industries, its nuclear properties in the pure metallic form make it highly desirable for nuclear applications. Zr is highly corrosion resistant and has a high melting point (2128 K) with a low thermal neutron absorption cross-section of ZrσT = 0.18 b, which is significantly smaller than that of hafnium HfσT =115 b) (Speer, 1982), an element that coexists with Zr in nature. This makes Zr ideal for nuclear cladding material and for an inert fuel matrix (Chadwick et al., 2011). Since unrefined Zr metal is more prone to corrosion in a high-temperature aqueous and steam environment (due to metallic and nonmetallic impurities), stringent quality standards have to be met to ensure optimal performance of the zirconium alloy in a nuclear environment. This requires zirconium alloys with a low neutron capture coefficient, high corrosion resistance with regard to nuclear coolants, high manufacturability, and cracking resistance of components during operation, as well as in the manufacturing process itself. While Zr for use in the nuclear industry needs to contain less than 100 ppm Hf, various aspects of metal procurement, purification, and production have to be taken into account to make zirconium alloy of the highest grade. The <100 ppm Hf requirement is likely to be replaced soon by an American equivalent, Zircaloy-4; or Russian equivalent, Zr-1%Nb. These zirconium alloys, which are already in use in nuclear reactors, contain Hf concentrations as low as 42 ppm and 80 ppm respectively (Nikulina and Malgin, 2008).
The main impurities found in nuclear-grade Zr are titanium (Ti), boron (B), cadmium (Cd), cobalt (Co), manganese (Mn), molybdenum (Mo), sodium (Na), magnesium (Mg), silicon (Si), carbon (C), hydrogen (H), chlorine (Cl), potassium (K), calcium (Ca), fluorine (F), phosphorous (P), oxygen (O), nitrogen (N), iron (Fe), nickel (Ni), chromium (Cr), copper (Cu), aluminum (Al), and hafnium (Hf). The most is known about the effects of O, N, H, C, Si, Al, Fe, Ni, and Hf. The presence of N, C, Al, and Ti results in decreased corrosion resistance (in high-temperature aqueous environments), while the presence of C, H, Si, Cl, P, and F results in decreased fracture toughness and, as a result, poor manufacturability and cracking resistance of parts. Oxygen improves the strength of zirconium alloys, while Fe improves corrosion resistance. Ni intensifies hydrogen absorption, which in turn decreases fracture toughness. The detrimental effects of impurities on the properties of zirconium alloys are synergistic.
Consequently, concentrations of impurities relative to Zr as well as to other impurities are important. The performance of zirconium alloys therefore depends not only on the thermonuclear properties, but also on the: microstructure, porosity, fracture toughness, and plasticity, as well as residual plasticity (Nikulina and Malgin, 2008).
The South Africa Nuclear Energy Corporation (NECSA) processes South African zircon ore via a plasma dissociated zircon (PDZ) process and reports that the following impurities are present after the plasma fluorination process: Hf, Si, Fe, Ti, Al, Cr, Mg, Ca, P, U, Th, Fe, Ti, Al, Cr, Mg, Ca, U, and Th. The impurities are present at this stage as oxide and fluoride complexes.
Once the abovementioned impurities have been removed, the production of pure Zr remains difficult, due to the presence of 0.5-3 wt% Hf impurity in the original ore (Belousova et al., 2002). The difficulty originates from the chemical similarities of Zr and Hf in both metallic and compound form. The most well-known methods of separation include extractive distillation (CEZUS Process, with a separation factor (SF) of approximately 2 (Banda and Lee, 2015) and solvent extraction (SX) using MIBK (SF approx. 7) or TBP (SF approx. 10) (Banda and Lee, 2015). Many alternative methods have been investigated and are summarized in the following paragraphs.
Fritz and Frazee (1965), applied reversed-phase chromatographic separation to solutions containing 250 and 50 pmole Zr and Hf, respectively, and successfully purified Zr to 0.001-0.002% Hf content. The quantitative separation was obtained using Teflon-6 as solid support and methyl isobutyl ketone, pre-equilibrated with 3 M thiocyanic acid, as the stationary phase. Zr was eluted with an ammonium thiocyanate-ammonium sulphate solution and Hf stripped from the column with an ammonium sulphate or sulphuric acid solution. Successful column separations were also achieved on solutions containing Zr to Hf ratios of 5, 25, and 100 to 1, with resulting impurity levels of 0.01% Hf (in Zr) and 0.01% Zr (in Hf).
Akl et al. (2001) demonstrated that with separation-flotation 0-20 χ 10-5 mol/L of both Zr and Hf could be completely separated using an aqueous solution of HCl at pH 2 containing Eriochrome Cyanine R (ECR) and an organic solution of the oleic acid surfactant HOL in kerosene. The suggested application of this technique was the separation and microdetermination of Zr and Hf in water samples.
Poriel et al. (2006), reported a selectivity (a) of >2.5 on a 27 mmol Zr and 11 mmol Hf mixed solution, with ligand-enhanced separation via ultra/nano-filtration membranes. The best results were obtained with the organic thin-film composite nanofiltration membrane Desal G10 (Osmonics). As EDTA-Hf complexes have a higher thermodynamic stability constant than Zr-EDTA complexes, ethylenediaminetetraacetic acid (EDTA) was used as depolymerization agent. The mechanism of separation is the transport of the EDTA-Hf complexes, which form preferentially, across the membrane.
Smolik et al. (2009) utilized 6.5 g Diphonix® resin (containing diphosphonic, sulphonic, and carboxylic acid groups) for the separation of Zr from Hf in aqueous sulphuric acid medium (0.5 M) containing 6.38 g/l Zr and 0.154 gl Hf. They found that a reduction in temperature (22°C to 5°C) decreased the degree of separation while lower column flow rates increased the extent of separation. Under these conditions, a 10-fold decrease (from 2.4% to 0.24%) of Hf in Zr was achieved with 45% recovery of Zr. The results indicated that Diphonix® resin is suitable for the separation of Zr from Hf by a continuous ion exchange process.
Li et al. (2011) studied the combination of combustion synthesis and molten-salt electrorefining. Zr metal was produced containing <70 ppm Hf through separation by exploitation of the differences in the thermodynamic stabilities of Zr and Hf fluorides. Firstly, ZrSi powder was produced by magnesiothermic reduction of ZrSiO4 in an argon atmosphere at a pressure of 2.5 MPa. The ZrSi was then arc melted and cast into granules for use as raw material in the electrorefining process.The electrorefining cell consisted of a high-purity LiCl-KCl eutectic (chloride electrolyte; 58.5-41.5 mol%, Tm= 352°C) and 5 wt% ZrF4 (electrorefining initiator, Hf <0.1 wt%). It had a calculated efficiency of 57%.
As can be seen from the above examples, most techniques are limited to analytical use and, however successful, some are not as well suited and easily implemented as others. The purpose of this study was to investigate the separation of Zr and Hf by means of SX, specifically focusing on the interaction of the organophosphorus extractant Ionquest 801 with feed concentrations (>1 g/l ZrCl4 containing 3 wt% HfCl4) that exhibits extraction behaviour approximately representative of more concentrated (industrial) feed streams (>10 g/l ZrCl4 containing 3 wt% HfCl4). The influence of various additives as a means to reverse the obtained selectivities was also studied.
Materials and methods
Materials and general procedure
Deionized water (pH 7.5) was used throughout (Millipore Milli-Q Plus® Q-pack CPMQ004R1) and all reagents were of analytical grade (see Table I). An aqueous feed was prepared by dissolving 1 g/l ZrCl4 plus 3 wt% HfCl4 in HNO3 or H2SO4 and ageing for 24 hours prior to extraction. The solvent consisted of cyclohexane (diluent), 5% v/v 1-octanol (modifier) and Ionquest 801 at a concentration of 5 wt% relative to the diluent. The two phases were contacted for 60 minutes in polypropylene containers at temperatures of 26 ± 4°C at an organic to aqueous (O/A) volume ratio of unity using a batch extraction set-up with a mechanical agitator oscillating at 330 r/min. All extractions were done in triplicate and the average values noted with a 10% relative error. The Zr and Hf concentrations of the feed were determined before and after extraction using ICP-OES (Thermo Scientific iCAP 6000 Series spectrometer and CETAC ASX 520 Autosampler).
Methods
Influence ofHNO3 concentration
Using the general procedure described above, the influence of the HNO3 concentration on the extraction performance of Ionquest 801 was determined at [HNO3] = 0.01-5.0 M.
Influence oflonquest 801 concentration
Subsequently, the extraction performance of Ionquest 801 was determined at E:MZr ratios from 0.1:1 to 20:1 in both 0.7 and 3 M HNO3.
Influence of carboxylic acids
The extraction performance of Ionquest 801 was determined with additions of 0.1 M tartaric, oxalic, mandelic, and citric acid in 0.7 M HNO3 and at an E:MZr ratio of 5:1.
Influence ofcarboxylic acids in the presence ofH2O2
The extraction performance of Ionquest 801 was determined with the addition of 6 w% H2O2 and 0.1 M tartaric, oxalic, mandelic, and citric acid, in 0.7 M HNO3 and at an E:MZr ratio of 5:1.
Influence of saltlng-outagents
After optimization of the acid and extractant concentration, the extraction performance of Ionquest 801 was determined with additions of 1.5 M NaCl, NaNO3, Na2SO4, and NaClO4.H2O in 0.7 M HNO3 and at an E:MZr ratio of 5:1.
Influence of SO4-2 concentration in 0.35 Μ H2SO4
The extraction performance of Ionquest 801 was determined with the addition of 0-1.05 M Na2SO4 in 0.35 M H2SO4 at an E:MZr ratio of 5:1.
Influence of H2O2 in 0.35 Μ H2SO4
The extraction performance of Ionquest 801 was determined with additions of 0.6, 1.2, 1.8, 2.4, and 3 wt% H2O2 in 0.35 M H2SO4 and at an E:MZr ratio of 5:1.
Influence of E:MZr in 0.35 Μ H2SO4
The extraction performance of Ionquest 801 was determined at E:MZr ratios of 1:1 to 10 :1 in 0.35 M H2SO4.
Results and discussion
Influence of HNO3 concentration
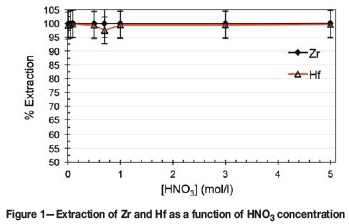
From Figure 1, it is clear that the extraction of both metal complexes remained above 97% over the entire acid concentration range (0-5 M). Although the unselective and high extraction attained could be ascribed to the excess of Ionquest 801 (5 wt%) used, the results clearly show that Ionquest 801 can extract the metal complexes of both Zr and Hf and is thus suitable for further study. It is important to note, however, why this system was chosen to start with. The choice of acid, extractant, and ageing are explained in the paragraphs that follow.
The following are important aspects to consider when optimizing an SX system: type of extractant, ageing, type of acid, and the metal concentration in the feed. Lee et al. (2015) studied two SX systems, consisting of Cyanex 272 and D2EHPA (each 0.01-0.07 M) in 2, 4 and 6 M H2SO4 solutions for different Zr/Hf ratios. They found that both systems selectively extracted Hf over Zr, irrespective of the Zr:Hf ratio (1:1-100:1) with all feed solutions containing 0.2 g/l Hf and the corresponding amount of Zr. Studies have shown that Ionquest 801 not only has a similar structure to D2EHPA, but also similar extraction behaviour. Peppard et al. (1969) found that Ionquest 801 showed significantly higher extraction of U(VI) compared to D2EHPA. In addition, high extraction has been observed in less acidic environments, with less emulsion formation and improved stripping. Furthermore, Ionquest 801 was used successfully for the separation of higher valence elements or heavy rare earth elements (REE) as well as for group separations of mixtures containing large amounts of heavy REE (Otu and Westland, 1990). It is for these reasons that the separation of Zr and Hf was investigated using Ionquest 801.
The reason for ageing the acidic aqueous feed solution was as follows. Zr/Hf and Ta/Nb systems are similar in that they are neighbouring groups in the same period of the periodic table of elements. While the speciation of these complexes in solution is unknown, research on the SX of the ammonium hexafluorides (NH4Ta(Nb)F6) of tantalum (Ta) and niobium (Nb) with D2EHPA and PA in H2SO4 medium has clearly shown the influence of time on extraction (De Beer et al., 2014). Although D2EHPA in H2SO4 medium exhibited preferential extraction of Ta over Nb, as in Hf extraction over Zr with similar conditions as described above, the amount extracted (%Ta) was stable for only 3.5 hours after acidification of the feed before decreasing from 96%Ta, to 30%Ta after 24 hours. In view of the similarity between the Ta/Nb and the Zr/Hf systems, all feed solutions in this study were hence aged for 24 hours before contact with the solvent. This ensures that the feed (solvated complexes) speciation is representative of leach liquors at chemical equilibrium.
The literature indicates that the use of the more corrosive H2SO4 is favourable. However, HNO3 was chosen to determine whether the manipulation of the SX system with salting-out agents could lead to acceptable extraction values. This would increase safety and applicability to other technologies, e.g. membrane-based solvent extraction (MBSX). The study was conducted on a macroscopic scale with respect to metal concentrations, conditions that would conceivably be encountered on an industrial scale. Hence feed concentrations >1 g/l (ZrCl4) containing 3-5 wt% HfCl4 (relative to ZrCl4) were used and assumed to exhibit extraction characteristics representative of more concentrated (industrial) feed streams.
Influence of Ionquest 801 concentration
As no substantial selectivity was obtained towards either complex, irrespective of the concentration of the HNO3 (see Figure 1), the influence of Ionquest 801 concentration on the extraction was investigated at both low (0.7 M HNO3) and high (3.0 M HNO3) acidities. The results are presented in Figures 2 and 3 respectively.
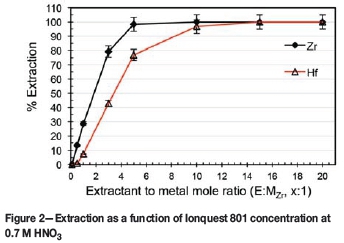
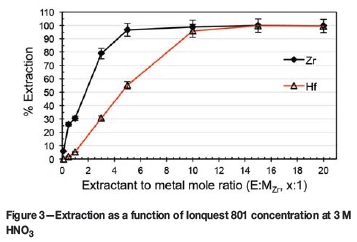
At 0.7 M HNO3, it is clear that there was an initial near-linear increase in extraction with increasing E:MZr ratio. It is also apparent that Ionquest 801, at low E:MZr ratios, preferentially extracted Zr, attaining a maximum Zr extraction (near 100%) at an E:MZr ratio of 5:1. Only when the E:MZr ratio reached 15:1 was complete extraction of Hf observed. This extraction remained practically unchanged for Zr at both acidities, with Hf extraction at an E:MZr ratio of 5:1 increasing from 55% at 3 M to 77% at 0.7 M. Wang and Lee (2014) reported similar findings using PC-88A (the same compound as Ionquest 801, obtained from Cytec Canada) in HNO3. Varying the PC-88A concentration at 2 M HNO3 produced a graph exhibiting the same characteristics as those in Figures 2 and 3. Zr extracts preferentially, as shown by the steeper slope, with Hf extraction following roughly the same trend but with lower extraction, until a suitable extractant concentration is reached and both metals extract completely. Direct comparison is, however, not possible as the feed concentrations differed from this study (0.2 g/l Zr + 0.2 g/l Hf vs. 1 g/l ZrCl4 + 3 wt% HfCl4). As a higher selectivity is preferred, the conditions of 0.7 M HNO3 with an E:MZr ratio of 5:1 were chosen for further optimization.
Influence of carboxylic acids
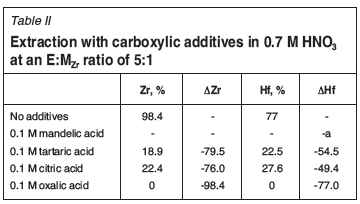
Table II shows the effect of four different acidic additives to Ionquest 801 on the extraction of Zr and Hf. The addition of mandelic acid to the 0.7 M HNO3 feed resulted in the complete precipitation of both metal complexes (99.9%Metal precipitation in feed). Tartaric and citric acid both suppress Zr extraction more than Hf, by a factor of 1.46 and 1.54 respectively. The addition of oxalic acid to the feed rendered both complexes unextractable. These results are surprising, as carboxylic acids and mixtures thereof are often used as extractants themselves (Lee et al., 2004), which would suggest that addition of these acids would increase extraction. The mechanism reported by Lee et al. (2004) was the solvation of the metal complex by two molecules of Versatic acid 10. This would most likely be dependent on the variety of extraction mechanisms present and competition between them. The use of carboxylic acids does, however, seem to be beneficial considering the outcome of the study conducted by Das etal. (1981). They used radio tracers (175,181Hf, 95Zr) to optimize a SX system consisting of 0.08 N H2SO4, 0.1 M carboxylic acid, 6 wt% H2O2, and 0.01 M D2EHPA in carbon tetrachloride (CCl4). After extraction with mineral acids, the extraction performance of D2EHPA with organic acids was tested. They found that all the carboxylic acids tested resulted in increased extraction relative to the mineral acids previously tested, including HCl and HF. In their study, the extractions on the carboxylic acids were carried out in the absence of other acids. Cumulatively, each additive resulted in an Hf-selective SX system, which could be of significance if the principles are applicable to high feed concentrations.
Influence of carboxylic acids in the presence ofH202
The experiments presented above were repeated with the addition of 6% H2O2 (Table III) to the aqueous phase. With H2O2 added, tartaric, mandelic, and citric acid formed organic emulsions after contact with the solvent (organic phase). Only oxalic acid produced results that were similar to those obtained without the addition of H2O2 (see Table II).
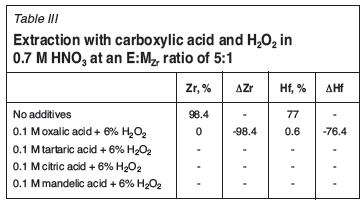
Das et al. (1981) found that the addition of H2O2 decreases the extraction of Zr, while the Hf extraction remains unchanged. This effect was most prominent for HClO4 (%Zr/%Hf = 15/63) and H2SO4 (%Zr/%Hf = 7/72). They ascribed the inability of D2EHPA to extract Zr in the presence of H2O2 to the fact that at low acidities, the tendency of Zr to polymerize is greater than that of Hf. The proposed metal species formed under these conditions is a highly aggregated complex consisting of Zr metal atoms connected via stable oxygen bonds as bridges between the metal centres. These bridges lead to the formation of Zr aggregates that are unextractable by the extractant. Considering the strong similarities between Zr and Hf, it is likely that smaller Hf aggregates do form, but to a lesser extent. It is likely that these Hf aggregates lead to the formation of emulsions and third phases in the solvent, as the higher feed concentrations enable the formation of larger quantities thereof. The precipitation in the feed, however, is assumed to be due to hydrolysis after the aggregation process is saturated (see section below on the influence of H2O2 where the extent of the feed precipitation was determined). Thus it seems that the aqueous phase can accommodate both aggregates, with the much smaller Hf aggregates capable of migration to the solvent through extraction with Ionquest 801. The experimental confirmation of this falls, however, beyond the scope of this paper.
Influence of salting-out agents
It is known that reaction additives can have a significant effect on the speciation (and hence extraction characteristics) of a metal complex. This principle dates as far back as Le Chatelier, who stated that a system will rearrange itself (partially) to counteract the change in pressure, volume, temperature, or concentration imposed upon it (Kotz and Treichel, 2003). Thus as soon as ions are introduced, the chemical equilibria change, which can be used to influence the SX system. This was demonstrated by Das et al. (1981), who studied the extraction of radio tracers (175,181Hf, 95Zr)
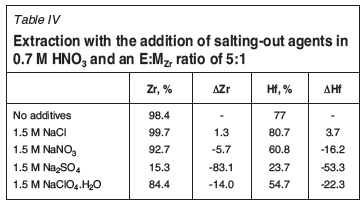
with D2EHPA in the presence of various mineral acids. The presence of SO-42 ions in the feed enables Zr to form strongly bonded complexes compared to other ions (NO3-, Cl-, ClO-4), resulting in a decrease in extraction. The best Hf selectivity was reported using the mineral acids H2SO4, HClO4, and HNO3. As dissociated acids release protons and anions, the addition of salting agents might produce the same effect if it is assumed that the acids dissociate completely. To study this, the influence of NaCl, NaNO3, Na2SO4, and NaClO4 was evaluated.
It is clear from Table IV that the best results in terms of an inverse Hf selectivity were achieved with the addition of 1.5 M Na2SO4. It is interesting to note that an increase in NO-concentration from 0.7 M HNO3 to 2.2 M (0.7 M HNO3 + 1.5 M NaNO3) resulted in a suppression of Hf extraction (A%Hf= -16.2) by a factor of 2.84, compared to Zr extraction (A%Zr= -5.7). Conversely, the addition of 1.5 M Na2SO4 to the feed suppressed Zr extraction over Hf extraction by a factor of 1.56, even in the presence of 0.7 M NO3-. As nitrate anions suppress Hf extraction preferentially, the use of nitrate was excluded from this study.
Influence of SO42" concentration in 0.35 Μ H2SO4
In view of the above results it was decided to investigate the characteristics of an H2SO4-based system instead of the hitherto-used HNO3 system. To maintain the same H+ concentration (0.7 M) the H2SO4 was used at 0.35 M. Considering the dominating strength (in terms of anion displacement and complex stability) of sulphate anion complexes and the polymerization characteristics of Zr and Hf at low acidities, ascribing the extraction or lack thereof to any single cause is difficult and beyond the scope of this study. The nature and extent of the polymerization of these metals at low acidities are unknown, and thus the extraction percentage will be considered as the outcome of the cumulative effects of the phenomena present. As can be seen from Figure 4, the presence of H2SO4 resulted in an inverse selectivity in favour of Hf. Extraction of the metal complexes at these conditions led to an extraction difference of roughly 13% in favour of Hf, with a Hf extraction of 39% and Zr 26%. It is clear that increasing the sulphate concentration above 0.35 M, as dissociated Na2SO4, decreased the separation and recovery. The resultant drop in extraction for Hf (from 0.35 M SO42- to 0.7 M) is almost double that of Zr, as the equilibrium driving forces alter the speciation.
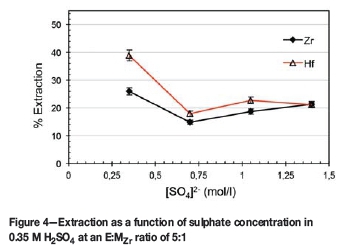
This is in good agreement with the results of Banda et al. (2013). Although the experimental conditions varied somewhat in that the feed contained 0.2 g/l Zr and Hf, and D2EHPA was used as extractant, as previously mentioned, D2EHPA and Ionquest 801 have similar extraction behaviours. It is clear from the results of Banda et al. that increased extraction and separation is attained at low acidities (<2 M H2SO4) rather than high acidities. Note that at low acidities (0.5 M H2SO4) the extraction of Hf is roughly double that of Zr, even for the feed (1 g/l ZrCl4 + 3 wt% HfCl4) used with Ionquest 801 at 0.35 M H2SO4.
From a structural analysis point of view, Hu et al. (2013) combined high-energy X-ray scattering (HEXS), Raman spectroscopy, and mathematical manipulations such as Fourier transformations and Gaussian curves to obtain partial pair distribution functions (PDFs). These PDFs show only those correlations involving Zr (specifically including those with complexed species, solvent molecules, and other solute ions) and represent electron counts in Zr ion correlations, enabling possible species to be characterized and elucidated according to relative intensities. Hu et al. (2013, Figure 4) plotted changes in the integrated intensities of selected PDF peaks (3.56, 4.97, and 6.7 À) as a function of the total sulphate concentration in solution. All three peaks increase in intensity up to [SO42-] = 0.5 M with the peaks at 4.97 and 6.7 À having roughly the same intensity and that of 3.56 À being 2-2.5 times more intense. Hu et al. (2013) interpret this as follows: 'This behavior is suggestive of changing average cluster size across the series, increasing with increasing sulfate concentration up to 0.5 m in sulfate, and slowly decreasing with higher sulfate concentration.' This then lends merit to the notion that these larger complex clusters may be harder to extract and is likely the reason why extraction is more successful at low acidities and low sulphate concentrations.
Influence of H2O2 in 0.35 Μ ΗβO4
Addition of >2.4% v/v H2O2 (as the only additive) to the 0.35 M H2SO4 feed led to the formation of aqueous precipitates (after 24 hours ageing). Addition of <1.8% v/v H2O2 led to the formation of emulsions after solvent contact. The extent of precipitation was determined in the range of 2.4-4.8% v/v H2O2. The percentage Hf precipitated (11.8-40.3%) is roughly double that of Zr (5.2-25%). It is clear that Hf is hydrolysed more readily than Zr, which is considered to be highly aggregated in this environment. This lends credibility to the notion that at these low acidities the speciation of Zr and Hf differs. Thus the capability of H2O2 to influence the system and metal complex speciation is a dominant process that should be studied further. The formation of stable aggregates, monomers, and dimers (whether from H2O2 aggregate formation or sulphate-induced polymerization) provide an opportunity for increasing the selectivity of a process, as these might be harder to extract via certain mechanisms. For example, ion exchange requires the exchange of ions between the extractant and the complex, which would not occur if the complex were too stable for this process. The processes of polymerization and aggregation, as well as the extent thereof, are not well documented for Zr and Hf systems. However, the data presented gives some support to these principles.
We refer again to the system referenced throughout this article. Das et al. (1981) used tartaric acid in their system (0.08 N H2SO4, 0.1 M tartaric acid, 6 wt% H2O2, and 0.01 M D2EHPA in CCl4) and observed the preferential extraction of 175,181Hf over 95Zr (radio tracers) with extraction percentages of 100% and 1-2% respectively. This shows that the suppressive effects of H2O2 and sulphate anions can restrict the extraction of Zr, while the tartaric acid seems to facilitate Hf extraction. These principles seem to be plausible and warrant further investigation.
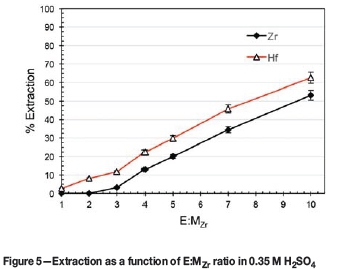
Influence of E:MZrin 0.35MN2SO4
To improve the recovery and re-evaluate the selectivity at the new parameters obtained from the experiment on the influence of SO42- concentration, the extraction performance of Ionquest 801 was determined over a wider range of E:MZr values.
A comparison of the extraction values in Figure 4 and Figure 5 shows that the selectivity of the process decreased slightly from A%E= 13% (E:MZr = 5:1 in 0.35 M H2SO4 with no addition of Na2SO4) to A%E= 10% (E:MZr> 3:1 in 0.35 M H2SO4). The difference in maximum extraction values at corresponding points (E:MZr = 5:1) of these two figures are most likely due to the values of Figure 5 having been obtained at lower extraction temperatures. From this data it is evident that an E:MZr ratio 10:1 is already sufficient, with more than 60% Hf extraction.
Conclusions
When using HNO3, higher extraction of Hf was attained at an E:MZr ratio of 5:1 in 0.7 M HNO3 than in 3 M HNO3, with an extraction of 77%Hf and 55.2%Hf respectively. Zr extraction remained constant (>97%Zr) at both acidities. Conditions favouring Hf extraction (E:MZr ratio of 5:1 in 0.7 M HNO3) were therefore set as the basis of the SX system and further investigated. Addition of 1.5 M Na2SO4 to the system suppressed Zr extraction preferentially (A%Zr= -83.1) to a value of 15.3%Zr, while Hf extraction decreased to 23.7%Hf (A%Hf= -53.3). Addition of 1.5 M NaNO3 to the system supressed Hf extraction preferentially (A%Hf= -16.2) to a value of 60.8%Hf, while Zr extraction merely decreased to 92.7%Zr (A°/oZr= -5.7). This indicated that NO- ions are detrimental to Hf extraction and should be excluded from the SX system, while SO4-2 ions are detrimental to Zr extraction and could be used to supress Zr extraction preferentially. Nitrate ions were excluded, while maintaining the acidity and sulphate ions, by addition of protons and sulphate ions as H2SO4 and Na2SO4. The best salting-out agent tested was Na2SO4, which was used for further optimization. Carboxylic acids have a suppressive effect (in 0.7 M HNO3) on extraction, favouring Hf extraction. However, this may change significantly with the use of H2SO4 and addition of H2O2. It was shown that low acidity (0.7 M H+ with the use of 0.35 M H2SO4) resulted in the highest selectivity and recovery of Hf. However, the use of H2O2 led to feed precipitation or emulsions forming in this batch SX process. The use of a hollow-fibre membrane (MBSX) would avoid emulsions, enabling the use of H2O2. In addition, the influence of higher E:MZr (> 5:1) ratios was investigated on the optimized system containing only 0.35 M H2SO4. An E:MZr ratio of 10:1 was sufficient to obtain >50% extraction of the solute of interest with an trivial change in selectivity. Acidities below 1.5 M H+ (via addition of HCl) and anion concentrations below 0.35 M [SO4-2] could be further investigated to maximize Hf recovery and possibly increase selectivity.
References
Akl, M.A., Kabil, M.A., Abdallah, A.M., and Ismail, D.S. 2001. Use of eriochrome cyanine R for separation-flotation and microdetermination of hafnium and zirconium in real samples. Separation Science and Technology, vol. 36, no. 12. pp. 2747-2760. [ Links ]
BANDA, R. and LEE, M.S. 2015. Solvent extraction for the separation of Zr and Hf from aqueous solutions. Separation and Purification Reviews, vol. 44, no. 3. pp. 199-215. [ Links ]
Banda, R., Min, S.H., and Lee, M.S. 2013. Selective extraction of Hf(IV) over Zr(IV) from aqueous H2SO4 solutions by solvent extraction with acidic organophosphorous based extractants. Journal of Chemical Technology and Biotechnology, vol. 89, no. 11. pp. 1712-1719. [ Links ]
Belousova, E., Griffin, W.L., O'Reilly, S.Y., and Fisher, N. 2002. Igneous zircon: trace element composition as an indicator of source rock type. Contributions to Mineralogy and Petrology, vol. 143, no. 5. pp. 602-622. [ Links ]
Chadwick, M.B., Herman, M., ObloSnský, P., Dunn, M.E., Danon, Y., and Kahler, A.C. et al. 2011. ENDF/B-VII.1 Nuclear Data for Science and Technology: Cross Sections, Covariances, Fission Product Yields and Decay Data. Nuclear Data Sheets, vol. 112, no. 12. pp. 2887-2996. [ Links ]
Das, N.R., Nandi, B., and Bhattacharyya, S.N. 1981. Sequential separation of hafnium, zirconium and niobium from sulphuric acid medium using di(2-ethylhexyl) phosphoric acid as an extractant. International Journal of Applied Radiation and Isotopes, vol. 32, no. 4. pp. 205-209. [ Links ]
De Beer, L., Ungerer, M.J., Van der Westhuizen, D.J., and Krieg, H.M. 2014. The time dependent solvent extraction of Ta and Nb. Advanced Materials Research. http://www.scientific.net/AMR.1019.433 [ Links ]
Fritz, J.S. and Frazee, R.T. 1965. Reversed-phase chromatographic separation of zirconium and hafnium. Analytical Chemistry, vol. 37, no. 11. pp. 1358-1361. [ Links ]
Hu, Y-J., Knope, K.E., Skanthakumar, S., Kanatzidis, M.G., Mitchell, J.F., and Soderholm, L. 2013. Understanding the role of aqueous solution speciation and its application to the directed syntheses of complex oxidic Zr chlorides and sulfates. Journal of the American Chemical Society, vol. 135, no. 38. pp. 14240-14248. [ Links ]
Kotz, J.C. and Treichel, J.P.M. 2003. Chemistry and Chemical Reactivity. 5th edn. Thomson Learning. 997 pp. [ Links ]
Lee, H.Y., Kim, S.G., and Oh, J.K. 2004. Stoichiometric relation for extraction of zirconium and hafnium from acidic chloride solutions with Versatic acid 10. Hydrometallurgy, vol. 73, no. 1-2. pp. 91-97. [ Links ]
Lee, M.S., Banda, R., and Min, S.H. 2015. Separation of Hf(IV)-Zr(IV) in H2SO4 solutions using solvent extraction with D2EHPA or Cyanex 272 at different reagent and metal ion concentrations. Hydrometallurgy, vol. 152, no. 0. pp. 84-90. [ Links ]
Lí, H., Nersisyan, H.H., Park, K-T., Park, S-B., Kim, J-G., Lee, J-M., and Lee, J-H. 2011. Nuclear-grade zirconium prepared by combining combustion synthesis with molten-salt electrorefining technique. Journal of Nuclear Materials, vol. 413, no. 2. pp. 107-113. [ Links ]
Nikulina, A.V. and Malgin, A.G. 2008. Impurities and their effect on the structure and properties of zirconium parts in nuclear reactors. Atomic Energy, vol. 105, no. 5. pp. 328-339. [ Links ]
Otu, E.O. and Westland, A.D. 1990. Solvent extraction with organophosphonic mono-acidic esters. Solvent Extraction and Ion Exchange, vol. 8, no. 6. pp. 759-781. [ Links ]
Peppard, D., Mason, G., and Lewey, S. 1969. A tetrad effect in the liquid-liquid extraction ordering of lanthanides (III). Journal of Inorganic and Nuclear Chemistry, vol. 31, no. 7. pp. 2271-2272. [ Links ]
Poriel, L., Chitry, F., Pellet-Rostaing, S., Lemaire, M., and Favre-Réguillon, A. 2006. Zirconium and hafnium separation, Part 3. Ligand-enhanced separation of zirconium and hafnium from aqueous solution using nanofiltration. Separation Science and Technology, vol. 41, no. 13. pp. 2883-2893. [ Links ]
Smolik, M., Jakóbik-Kolon, A., and Poranski, M. 2009. Separation of zirconium and hafnium using Diphonix® chelating ion-exchange resin. Hydrometallurgy, vol. 95, no. 3-4. pp. 350-353. [ Links ]
Speer, J.A.C. and Cooper, B. J. 1982. Crystal structure of synthetic hafnon, HfSiO4, comparison with zircon and the actinide orthosilicates. American Mineralogist, vol. 67. pp. 804-808. [ Links ]
Wang, L.Y. and Lee, M.S. 2014. Separation of zirconium and hafnium from nitric acid solutions with LIX 63, PC 88A and their mixture by solvent extraction. Hydrometallurgy, vol. 150, no. 0. pp. 153-160. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2016. ISSN2225-6253. This paper was first presented at the, Nuclear Materials Development Network Conference 2015, Nelson Mandela Metropolitan University, North Campus Conference Centre, Port Elizabeth, South Africa.














