Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.12 Johannesburg Dec. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n12a14
PAPERS OF GENERAL INTEREST
The influence of water quality on the flotation performance of complex sulphide ores: Case study at Hajar Mine, Morocco
K. BoujounouiI; A. AbidiII; A. BacaouiI; K.El AmariIII; A. YaacoubiI
IFaculty of Sciences Semlalia, department of chemistry, BP 2390 Marrakech, Morocco
IIMining Institute of Marrakech (IMM), B.P. 2402, Marrakech, Morocco
IIILaboratoire Géoressources, Unité associée au CNRST (URAC 42), Faculté des Sciences et Techniques Marrakech,. Marrakech, Morocco
SYNOPSIS
As part of the process optimization project of CMG (Mining Company of Guemassa-Marrakech, Morocco), a preliminary study on the effect of water quality on the flotation of galena, sphalerite, chalcopyrite, and pyrrhotite was carried out using asymmetrical fractional factorial design. The multivariable analysis showed that of ten studied factors, six (Cu2+, Zn2+, Mg2+, Ca2+, SO42-, and PAX) have a significant influence on the flotation of these sulphide minerals. Graphical analysis showed that high concentrations of Cu2+ (7-14 mg/L) in synthetic process water increased the recovery of galena, chalcopyrite, sphalerite, and pyrrhotite. At low Cu2+ concentrations (0-7 mg/L), sphalerite was depressed. Zn2+ at low concentrations (0-20 mg/L) decreased the recovery of all studied minerals. However, at high concentrations (20-40 mg/L), an increase in chalcopyrite, sphalerite, and pyrrhotite recoveries was observed. Mg2+ (100-200 mg/L) decreased the recovery of galena, chalcopyrite, and sphalerite. Ca2+(1200-2000 mg /L) depressed sphalerite flotation. Sulphate ions (SO42-) enhanced recovery of all the studied minerals. Potassium amyl xanthate (PAX) promoted sphalerite recovery at high concentrations (10-20 mg/L).
Keywords: flotation, process water chemistry, complex sulphide ore, screening design.
Introduction
Mineral processing can be considered as one of the most intensive water-consuming processes. To reduce fresh water consumption, numerous research works have focused on the use of recycled process water. However, in flotation, recycling water can have adverse effects on the mineral separation. The content of organic reagents (frothers, collectors, depressants) as well as inorganic constituents (suspended matter, base metals, calcium, magnesium, sodium, sulphite, sulphate etc.) (Leavay etal., 2001; Johnson, 2003; Slatter et al., 2009) builds up and consequently affects the flotation performance (Lui et al., 1993; Leavay et al., 2001; Seke et al.,2006; Kelebek and Nanthakumar, 2007; Haran et al., 2008; Biçak et al., 2012; Ikumapayi et al., 2012). Generally, the hydrolyzed metallic ions in the alkaline recycled water form a hydrophilic precipitate (metal hydroxides, sulphates, or carbonates) on the mineral surfaces, resulting in the formation of a hydrophilic barrier that prevents adsorption of the collector (Senior and Trahar, 1991; Fornasiero and Ralston, 2006).
Cu2+in copper sulphate form is known as a sphalerite activator, and Zn recovery by flotation is enhanced by increasing concentrations of Cu2+ions in synthetic water (Biçak et al., 2012). Cu2+ can also enhance the recovery of galena, chalcopyrite, and pyrite (Coetzer et al., 2003). However, the effect of increasing copper concentration on flotation is not evident above pH 12 and below pH 5, where Cu(OH)3- and Cu2+are respectively the stable copper species (Fornasiero and Ralston, 2006).
The hydrophobicity of the sphalerite surface is controlled by the Zn(OH)2 formed (Prestidge et al., 1997; Fornasiero and Raston, 2006). For sphalerite in alkaline media, the surface Cu(OH)2 directly interacts with the xanthate (Leppinen,1990).
Cyanide ions used as depressant in a polymetallic ore can produce cupric ions by reaction with copper minerals and cause inadvertent activation of sphalerite. The activation process is enhanced at higher pH values (Seke et al., 2006; Rao et al., 2011).
The presence of Zn2+ in recycled water strongly depresses the recovery of sphalerite, and slightly depresses chalcopyrite and pyrite, but favours the recovery of galena (Coetzer et al., 2003). The use of zinc sulphate at alkaline pH decreases the recovery of galena due to coating by hydrophilic Zn(OH)2 (Trahar et al., 1997; Seke, 2005).
Ca2+and Mg2+are the most cited cations in the literature as precipitated species with a negative effect on mineral recoveries. Sphalerite recovery is significantly reduced in the presence of these ions; the decrease is more pronounced for Mg at pH 9 than for Ca at pH 12. This is due to the formation of the metal hydroxides on mineral surfaces (Lascelles et al., 2003). The adsorption of calcium and other metal ions in during flotation leads to the reduction of negative surface charges and consequently inhibits the adsorption of xanthate on the mineral surfaces (Ikumapayi et al., 2012).
Generally, the presence of SO42- in process water containing numerous species, including SO32- and Ca2+, in addition to dissolved iron, could form hydrophilic layers on the surface mineral (CaSO42- or CaSO32-) (Ikumapayi et al., 2012). Furthermore, a high concentration of SO42- ions has the same depressing effect by competing with collector molecules for adsorption on mineral surfaces (Wu et al., 2002; Lefévre and Fédoroff, 2006).
In Morocco, the semi-arid to arid climate makes water a limited and precious resource. According to data from the Haouz Tensift Basin Agency (HTBA), the Marrakech region of Morocco is characterized by low and irregular rainfall (250 mm/a) with a high evaporation rate (2500 mm/a). The Mining Company of Guemassa (CMG), located 30 km southwest of Marrakech, is affected by this issue of water shortage.
CMG uses a selective flotation flow sheet to produce a galena concentrate (with Aerophine A3418 using NaCN at pH of 11.3), then a chalcopyrite concentrate (with Aerophine A3418 at pH of 8.9), and finally a sphalerite concentrate (with potassium amyl xanthate; PAX, at pH of 12-12.5) from a complex polymetallic sulphide ore.
To reduce the consumption of fresh water and to test the possibility of using a single process water in the overall CMG flotation process, this study focused on the effect of simulated process water quality on the recovery of Pb, Cu, and Zn during the galena flotation step. The study considered the effect of various ions in the water, as well as the particle size of the feed. The goal was to achieve a better recovery and selectivity of galena over sphalerite, chalcopyrite, and pyrrhotite. The results will assist in the flotation optimization exercise by identifying the most important factors influencing galena recovery and the interactions between them.
In this study, flotation tests were carried out on a complex sulphide ore provided by CMG. The results were subjected to statistical analysis to assess the relative significance of the main factors affecting flotation performance as evaluated from the experimental results. The statistical design of the experiments allows a full study of the effects of all parameters on a given process and their optimization, providing maximum information from a minimum of experiments by implementing a simple mathematical model to represent the studied phenomenon (Box et al., 1978; Akhanazarova and Kafarov, 1982; Obeng et al., 2005; Napier-Munn, 2012; Ennaciri et al., 2014).
Material and methods
Methodology
To study the effect of water quality on the flotation of zinc, lead, and copper, the focus was on the parameters listed below and presented in Table I. These variables, with their respective ranges of values, were chosen on the basis of data from the literature and preliminary experiments.
> Water quality. Process water was synthesized from Marrakech drinking water. Cations and anions were added to simulate the composition of recycled water in the CMG zinc process. These ions are: Cu2+, Fe2+, Zn2+, Pb2+, Ca2+, Mg2+, SO42+, and PAX
• Marrakech drinking water was used during grinding. Once the pulp was introduced into the flotation cell, the desired synthesized water quality was adjusted by adding the salts of the relevant ions
• The grinding step was also performed using synthesized water directly
> Particle size - to distinguish the effect due to water quality and mineralogical mixing.
Screening of these ten studied parameters reduced the number tests required to 27 (Table I and II) and enabled the factors influencing the investigated responses: galena, chalcopyrite, sphalerite and pyrrhotite recoveries to be identified.
Material
Flotation tests were carried out on a representative sample of complex sulphide ore composed of 6.43% sphalerite (Sp: ZnS), 2.22% galena (Gl: PbS), 0.95% chalcopyrite (Cp: CuFeS2), 41.57% pyrrhotite (Po: Fe9S10), and 48.82% gangue (Gg) consisting mainly of silica, carbonates, and chlorides.
A sample of 120 kg was taken from the feed belt of the primary ball mill of the CMG flotation plant and crushed down to 2 mm using roll crushers in the laboratory of the Institute of Mines in Marrakech. The crushed sample was sieved at 2 mm and the undersize split using a riffle divider into 500 g batch samples for the flotation experiments. The batch samples were stored in vacuum sealed bags to prevent oxidation of the sulphide minerals.
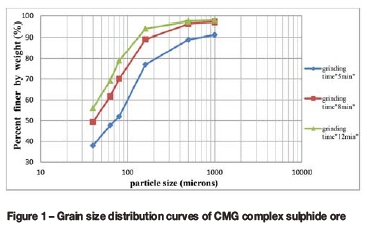
Prior to the flotation tests, samples of 500 g were milled in 250 ml of synthetic water using a Denver carbon steel ball mill of 9.5 litres internal volume. The size fractions studied in this work were d80= 200 μm, 100 μm, and 80 μm (Figure 1).
Flotation tests
Flotation tests were carried out in a Denver flotation cell of 1.5 litre capacity. Solid concentration was about 27% by weight, using synthetic water at the required quality.
The natural pH was about 7. NaOH was used as pH regulator for all tests to pH=11.3. Sodium cyanide (NaCN) was used as a depressing reagent for Sp, Cp, and Po for all tests at a specific addition of 350 g/t. Diisobutyl phosphinate (Aerophine 3418A) (40 g/t) and methyl isobutyl carbinol (MIBC) (40 g/t) were used as galena collector and frother respectively. The impeller rotation speed was a constant 700 r/min. The level of the pulp was constantly adjusted by the addition of synthetic water at the required quality. The flotation time was 10 minutes for each test, and the concentrate was recovered by manual scraping every 30 seconds. All concentrates and tails were filtered, dried, and weighed, and then analysed by atomic adsorption spectroscopy (AAS) for Cu, Pb, Zn, and Fe. Metal recoveries to the concentrates were calculated according to the following equation:
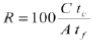
where R is the metal recovery; tcthe concentrate metal grade; tf the feed metal grade; C the concentrate weight, and A the feed weight.
Waters used for the tests were synthesized by using various salts: CaCl2 (97%), ZnSO4.7H2O (98%), and FeCl2.6H2O (97%) from Prolabo; Na2SO4 (99%) and PbCl2 (98%) from Fluka; Cu(NO3)2.3H2O (99.5%) from Merck; and Mg(NO3)2.6H2O (99-102%) from Panreac. Flotation reagents (Aerophine 3418A, PAX, and MIBC) were provided by CMG.
The effect of water addition was also studied. Water can be introduced at the grinding or at the flotation stage. Table II summarizes the designed experiments and operating conditions.
Experimental design
To assess the effect of each factor on the flotation of Pb, Cu, Zn, and Fe during the lead flotation step, the operating conditions of the tests were established according to an asymmetrical fractional factorial matrix. These kinds of multivariate experimental designs are powerful tools for the optimization of such procedures. Compared to traditional univariate approaches, based on the study of the effect of one variable at a time on the selected response, multivariate techniques allow the simultaneous evaluation of several factors with a minimum number of experiments. Furthermore, they provide additional data about the system, with the estimation of interactions between the influencing factors.
The screening experiment for several factors enables estimation of K factors in N experiments (K=10 and N= 27 in our case) with a variance of σ2/27. The model applied in this study is a polynomial empirical model of the first degree, which contains only the terms (bi) that will reflect the effect of each of the ten factors on the four responses: recovery of galena (R-Pb), of chalcopyrite (R-Cu), of sphalerite (R-Zn), and of pyrrhotite (R-Fe).
The model can be written as:
Y = b0 + b1A*(X1A) + b2A*(X2A) + b2B*(X2B) + b3A*(X3A) + b3B*(X3B) + b4A*(X4A) + b4B*(X4B) + b5A*(X5A) + b5B*(X5B) + b6A*(X6A) + b6B*(X6B) + b7A*(X7A) + b7B*(X7B) + b8A*(X8A) + b8B*(X8B) + b9A*(X9A) + b9B*(X9B) + b10A*(X10A) + b10B*(X10B)
where
Y: the studied response, which could be the recovery of lead (R-Pb), Y1: of copper (R-Cu), Y2: of zinc (R-Zn), Y3; or of iron (R-Fe), Y4
Xi: the investigated factor (i varies from 1 to 10)
A: the domain delimited by levels i and 2 of the factor Xi
B: the domain delimited by levels 2 and 3 of the factor Xi
biA: Xi effect in the domain A
biB: Xi effect in the domain B.
Tables I and II describe the approach of this study. The design matrix (fractional factorial), generated by the screening design resulted in the development of a series of 27 flotation tests (Table II). The experimental sequence was randomized in order to minimize the effects of the uncontrolled factors. The results were analysed using Nemrodw Software (New Efficient Methodology for Research using Optimal Design from LPRAI, Marseille, France) (Mathieu et al., 2000). The interpretation of the coefficients and the main effects of factors (bi), was performed from statistical tests on the coefficients using Student's test (tα/2,d).
Results and discussion
The results of the flotation tests conducted according to the experiments in Table II are presented in Figures 2-5 and Tables II and III. The standard errors calculated for the responses (R-Pb), (R-Cu), (R-Zn), and (R-Fe) were 3.215, 4.10, 2.240, and 3.338 respectively.
Table II shows that despite the flotation conditions (reagents, depressant, pH etc.) being favourable for good lead recovery and selectivity over Cu, Zn, and Fe, the flotation responses are affected by the quality of the process water. Lead recovery seems to be inversely related to the flotation recoveries of the other elements. This could be due to the composition of the process water, where dissolved species could depress galena and/or activate the other mineral phases. Their effects could be assessed from Figures 2-5, which are useful for identifying the statistically significant factors at level 99% (p-value < 0.01) and 95% (p-value < 0.05) (Table III). The limits of significance are represented by dashed lines in Figures 2a-5a, which depict the differences in the weight of the different levels for each response. Nonsignificant effects are those located between the two limits of significance.
From these figures, the more important factors influencing Pb, Cu, Zn, and Fe recoveries during the lead flotation step were derived and are presented in Table III. The effect of Cu2+ is clear on the responses of R-Pb, R-Cp, R-Sp, and R-Po. Increasing the Cu2+concentration from 7 to 14 mg/L (B2 to B3) has a positive effect on the recovery of all studied minerals. It also affects the selectivity for galena over chalcopyrite, sphalerite, and pyrrhotite. This selectivity could be enhanced at 0-7 mg/L Cu2+(B1 to B2), where a negative effect on the sphalerite recovery was observed.
The positive influence of high concentrations of Cu2+ on the recoveries of Ga, Sp, and Po could be due to the adsorption of Cu2+, Cu(OH)2, and Cu(OH)3- on the surfaces of these minerals (Prestidge et al., 1997; Fornasiero and Ralston, 2006; Chandra and Gerson, 2009). Low concentrations of Cu2+ depress only the recovery of sphalerite. This depressing effect might be due to weak absorption of copper onto the sphalerite surface due to competition with Cu2+ for adsorption sites (Deng et al., 2013). Moreover, the cyanide ions could cause inadvertent activation of sphalerite by cupric ions produced by the action of cyanide on copper minerals (Seke and Pistorius, 2006; Rao et al., 2011).
Zinc ions at concentrations from 0 to 40 mg/L (D1 to D3) have a negative effect only on the recovery of galena, but this negative effect is very pronounced from 0 to 20 mg/L (D1 to D2) on the recoveries of galena, chalcopyrite, sphalerite, and pyrrhotite. However, at Zn2+ concentrations from 20 to 40 mg/L (D2 to D3), the effect becomes positive except for galena. Both recovery and selectivity of galena over the other minerals are poor at zinc concentrations up to 40 mg/L.
The positive effect of Zn2+ could be due to the formation of hydrophobic precipitated species on the mineral surfaces (Znn-xCux.xZn(OH)2(surface), for example). Generally, Zn2+ions (as zinc sulphate) in alkaline conditions are used to depress sphalerite to increase lead/zinc selectivity.
High concentrations of Ca2+ in the synthesized water (1200-2000 mg/L) (F2 to F3) depress sphalerite and have no notable depressant effect on the other minerals, especially galena. The presence of Ca2+ might be considered beneficial within the lead circuit.
Mg2+at high concentrations (100-200 mg/L) (G2 to G3) has a very important negative effect on galena, chalcopyrite, and sphalerite recoveries, but no effect on pyrrhotite. The Mg2+ concentration in water process in the lead circuit must therefore be controlled.
The depressive effect of Ca2+ and Mg2+ could be due to the formation of hydrophilic layers such as CaCO3on the mineral surfaces in alkaline conditions, which prevents the adsorption of the collector onto mineral surfaces (Lascelles et al., 2003; Deng et al., 2013; Ikumapayi et al., 2012).
Sulphate ions (SO42-) from 200 to 6000 mg/L (H1 to H3) have a significant positive effect on the recovery of all studied minerals, consequently affecting the lead selectivity. This positive effect is weak from 200 to 1500 mg/L SO42- (H1 to H2) on the recoveries of galena, chalcopyrite, and sphalerite.
The positive effect of sulphate on recoveries could be due to the formation of heavy metal sulphite salts, which are slightly soluble in water (e.g. PbSO4, Ks = 1.8*10-8at 25°C in pure water), on the mineral surfaces.
High concentrations of PAX (1020 mg/L) in synthesized water (I1 to I2) promote sphalerite recovery. The positive effect of PAX is due to the formation of Cu(I)-xanthate and adsorption of dixanthogen (when Cu2+concentration is low) on the surface of the sphalerite (Leppinen, 1990; Popov and Vucinc, 1990). Xanthates are widely used in sulphide mineral flotation, especially in the selective flotation of sphalerite (Finkelstein, 1997).
The particle size (d80from 200 to 80 μm) (J1 to J3) has a positive effect on the recovery of all studied minerals. These effects are more pronounced between 200 to 100 μm (J1 to J2). Decreasing the particle size promotes the recovery of all studied minerals and affects the lead selectivity over Cu, Fe and Zn, due to the fact that the smaller the particle size, the greater the probability of adhesion to the air bubbles (Jowett et al., 1980), and the less the probability of detachment (Holtham et al., 1991).
Conclusions
Batch-scale flotation tests were performed on a complex Pb-Cu-Zn-Fe sulphide ore to investigate the influence of recycled water quality on the flotation of galena, chalcopyrite, and sphalerite during the lead flotation step. Screening experiments were conducted to study the effects of ten factors (Cu2+, Fe2+, Zn2+, Pb2+, Ca2+, Mg2+, SO42-, and PAX concentration, grain size, and water addition) on Pb, Cu, Zn, and Fe recoveries. The results showed that the influence of process water on lead flotation depends on its composition and concentrations of constituents.
> The addition level of recycled water (during the grinding or at the start of flotation) has no significant effect on the flotation of the studied minerals
> A small particle size enhances the recoveries of all the minerals studied
> Sulphate ions (SO42-) also have a positive effect on recoveries, but the domains of influence varies from one mineral to another
> High concentrations Cu2+increase the recovery of the} all studied minerals. Cu2+ has a depressing effect of sphalerite at low concentrations
> Zn2+ at low concentrations has a depressant effect on the recovery of all the mineral phases, but at high concentrations improves the recovery of chalcopyrite, sphalerite, and pyrrhotite
> Mg2+depresses galena, chalcopyrite, and sphalerite at the high concentrations
> Ca2+has a depressant effect on sphalerite at concentrations
> Potassium amyl xanthate at high concentrations enhances sphalerite recovery.
These factors, with their ranges of influence, will be the subject of further investigations to determine the nature of the interactions between them and their effects on recoveries. An optimization study will be carried out to determine the parameters that have the greatest influence on recoveries.
Acknowledgements
The authors thank the Mining Company of Guemassa for providing the sulphide ore sample and the flotation reagents, and Reminex Society for the chemical analyses.
References
Akhanazarova, S. and Kafarov, V. 1982. Experiment Optimization in Chemistry and Chemical Engineering. Mir Publishers, Moscow. [ Links ]
Bicak, O., Ekmekci, Z., Can, M., and Ozturk, Y. 2012. The effect of water chemistry on froth stability and surface chemistry of the flotation of a CuZn sulfide ore. International Journal of Mineral Processing, vol. 102-103. pp. 32-37. [ Links ]
Box, G.E.P., Hunter, W.G., and Hunter, J.S. 1978. Statistics for Experiments. Wiley, New York. [ Links ]
Chandra, A.P. and Gerson, A.R. 2009. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Advances in Colloid and Interface Science, vol. 145. pp. 97-110. [ Links ]
Coetzer, G., Du Preez, H.S., and Bredenhann, R. 2003. Influence of water resources and metal ions on galena flotation of Rosh Pinah ore. Journal of the South African Institute of Mining and Metallurgy, vol. 103, no. 3. pp. 193-207. [ Links ]
Deng, M., Liu, Q., and Xu, Z. 2013. Impact of gypsum supersaturated water on the uptake of copper and xanthate on sphalerite. Minerals Engineering, vol. 49. pp. 165-171. [ Links ]
Ennaciri, K., Ba£aoui, A., Sergent, M., and Yaacoubi, A. 2014. Application of fractional factorial and Doehlert designs for optimizing the preparation of activated carbons from Argan shells. Chemometrics and Intelligent Laboratory Systems, vol. 139. pp. 48-57. [ Links ]
Finkelstein, N.P. 1997. The activation of sulphide minerals for flotation: a review. International Journal of Mineral Processing, vol. 52. pp. 81-120. [ Links ]
Fornasiero, D. and Ralston, J. 2006. Effect of surface oxide/hydroxide products on the collectorless flotation of copper-activated sphalerite. International Journal of Mineral Processing, vol.78. pp. 231-237. [ Links ]
Haran, N.P., Boyapati, E.R., Boontanjai, C., and Swaminathan, C. 2008. Kinetics studies on effect of recycled water on flotation of copper tailings from Benambra Mines. Developments in Chemical Engineering and Mineral Processing, vol. 4, no. 3-4. pp. 197-211. [ Links ]
Holtham, P.N. and Cheng, T-W. 1991. Study of probability of detachment of particles from bubbles in flotation. Transactions of the Institution of Mining and Metallurgy Section C, vol. 100, Sep-Dec. pp. 147-153. [ Links ]
Ikumapayi, F., Makitalo, M., Johansson, B., and Rao, K.H. 2012. Recycling of process water in sulphide flotation: effect of calcium and sulphate ions on flotation of galena. Minerals Engineering, vol. 39. pp. 77-88. [ Links ]
Johnson, N.W. 2003. Issues in maximization of recycling of water in a mineral processing plant. Proceedings of Water in Mining 2003, Brisbane, 13-15 October. Publication Series No. 6/2003. Australian Institute of Mining and Metallurgy, Melbourne. pp. 239-245. [ Links ]
Jowett, A. 1980. Formation and disruption of particle-bubble aggregates in flotation. Fine Particles Processing. Somasundaran, O. (ed.). American Institute of Mining, Metallurgical and Petroleum Engineers. pp. 721-751. [ Links ]
Kelebek, S. and Nanthakumar, B.2007. Characterization of stockpile oxidation of pentlandite and pyrrhotite through kinetic analysis of their flotation. International Journal of Mineral Processing, vol. 84. pp. 69-80. [ Links ]
Lascelles, D., Finch, J.A., and Sui, C. 2003. Depressant action of Ca and Mg on flotation of Cu activated sphalerite. Canadian Metallurgical Quarterly, vol. 42, no. 2. pp 133-140. [ Links ]
Leavay, G.R.St., Smart, C., and Skinner, W.M., 2001. The impact of water quality on flotation performance. Journal of the South African Institute of Mining and Metallurgy, vol. 101, no. 2. pp. 69-75. [ Links ]
Lefévre, G. and Fédoroff, M. 2006. Sorption of sulfate ions onto hematite studied by attenuated total reflection-infrared spectroscopy: kinetics and competition with other ions. Physics and Chemistry of the Earth, Parts A/B/C, vol. 31, no. 10-14. pp. 499-504. [ Links ]
Leppinen, J.O. 1990. FTIR and flotation investigation of the adsorption of ethyl xanthate on activated and non-activated sulfide minerals. International Journal of Mineral Processing, vol. 30. pp. 245-263. [ Links ]
Liu, W., Moran, C.J., and Vink, S. 2013. A review of the effect of water quality on flotation. Minerals Engineering, vol. 53. pp. 91-100. [ Links ]
Lui, L., Rao, S.R., and Finch, J.A. 1993. Laboratory study effect of recycle water on flotation of a Cu/Zn Sulphide ore. Minerals Engineering, vol. 6, no. 11. pp. 1183-1190. [ Links ]
Mathieu, D., Nony, J., and Phan Tan Luu, R. 2000. Software NEMRODW. LPRAI-Marseille, France [ Links ]
Napier-Munn, T.J. 2012. Statistical methods to compare batch flotation grade-recovery curves and rate constants. Minerals Engineering, vol. 34. pp. 70-77. [ Links ]
Obeng, D.P., Morrell, S., and Napier-Munn, T.J. 2005. Application of central composite rotatable design to modelling the effect of some operating variables on the performance of the three-product cyclone. International Journal of Mineral Processing, vol. 76. pp. 181-192. [ Links ]
Popov, S.R., Vucinic, D.R., and Kacanic, J.V.1989. Floatability and adsorption of ethyl xanthate on sphalerite in an alkaline medium in the presence of dissolved lead ions. International Journal of Mineral Processing, vol. 27. pp. 205-219. [ Links ]
Prestidge, C.A., Skinner, W.M., Ralston, J., and Smart, R.C. 1997. Copper (II) activation and cyanide deactivation of zinc sulphide under mildly alkaline conditions. Applied Surface Science, vol. 108. pp. 333-344. [ Links ]
Rao, S.R., Nesset, J.E., and Finch, J.A. 2011. Activation of sphalerite by Cu ions produced by cyanide action on chalcopyrite. Minerals Engineering, vol. 24. pp. 1025-1027. [ Links ]
Seke, M.D. 2005. Optimization of the selective flotation of galena and sphalerite at Rosh Pinah Mine. PhD thesis, University of Pretoria, South Africa. [ Links ]
Seke, M.D. and Pistorius, P.C. 2006. Effect of cuprous cyanide, dry and wet milling on the selective flotation of galena and sphalerite. Minerals Engineering, vol. 19. pp. 1-11. [ Links ]
Senior, G.D. and Trahar, W.J. 1991. The influence of metal hydroxides and collector on the flotation of chalcopyrite. International Journal of Mineral Processing, vol. 33. pp. 321-341. [ Links ]
Slatter, K.A., Plint, N.D., Cole, M., Dilsook, V., De Vaux, D., Palm, N., and Oostendorp, B. 2009. Water management in Anglo Platinum process operations: effects of water quality on process operations. Abstracts of International Mine Water Conference, Pretoria, South Africa, 19-23 october. pp. 46-55. [ Links ]
Trahar, W.J., Senior, G.D., Heyes, G.W., and Creed, M.D. 1997. The activation of sphalerite by lead: a flotation perspective. International Journal of Mineral Processing, vol. 49. pp. 121-148. [ Links ]
Williams, S.R. and Phelan, J.M. 1985. Process development at Woodlawn Mines. Complex Sulphides, Processing of Ores, Concentrates, and Byproducts. Zunkel, A.D., Borman, R.S., and Wesely, R.J. (eds). AIME, New York. pp. 293-304. [ Links ]
Wu, C.H., Kuo, C.Y., Lin, C.F., and Lo, S.L. 2002. Modeling competitive adsorption of molybdate, sulfate, selenate, and selenite using a Freundlich-type multicomponent isotherm. Chemosphere, vol. 47, no. 3. pp. 283-292. [ Links ]
Paper received Mar. 2015
Revised paper received July 2015














