Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.12 Johannesburg Dec. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n12a13
PAPERS OF GENERAL INTEREST
The effect of sodium carbonate on the dispersion behaviour and froth flotation of a nickel ore
B. FengI, II; Q.M. FengII; Y.P. LuII; H.H. WangI
IJiangxi Key Laboratory of Mining Engneering, Jiangxi University of Science and Technology, Ganzhou, China
IISchool of Mineral Processing and Bioengineering, Central South University, Changsha, China
SYNOPSIS
The effect of sodium carbonate on the flotation performance of a nickel ore was studied and the mechanism investigated. The flotation results show that lizardite minerals in the ore interfere with the flotation of pentlandite, and the addition of sodium carbonate improves pentlandite flotation recovery. The pulp pH did not change with increasing sodium carbonate dosage. Drawing on the literature in this area, combined with the sedimentation test results, sieving test results, and infrared spectra study, it is proposed that carbonate ions, derived from sodium carbonate, interact with lizardite slime and change the interparticle force from attraction to repulsion, resulting in the removal of adhering slimes from pentlandite surfaces.
Keywords: sodium carbonate, nickel flotation, lizardite, pentlandite.
Introduction
Nickel deposits can be classified into two main groups: laterites and sulphides. Even though nearly 70% of nickel resources are contained in laterites, the bulk of production comes from sulphides due to the complex and high-cost processing required for laterite. The sulphide ores are universally treated by flotation, and the major gangue components include a host of MgO minerals, such as serpentine.
MgO minerals (e.g. serpentines) break readily. Thus grinding produces fines or slimes, particles less than approximately 10 μm. These gangue slimes can interfere with flotation by forming a coating on the pentlandite surfaces (Edwards et al., 1980; Pietrobon et al., 1997; Wellham et al., 1992). This has two consequences: dilution of the concentrate when pentlandite partially coated with fines remains floatable, and loss of pentlandite when extensively coated pentlandite becomes hydrophilic (Learmont and Iwasaki, 1984; Trahar, 1981). In order to improve the flotation of the pentlandite, sodium hexametaphosphate, sodium silicate, carboxymethyl cellulose (CMC), and other agents are used to disperse slime particles of MgO-type minerals from sulphide surfaces (Bremmell et al., 2005; Kirjavainen and Heiskanen 2007; Lu et al., 2011). Adsorption of these reagents on serpentine reverses the positive surface charge, so attraction forces between pentlandite and serpentine are eliminated (Bremmel et al., 2005).
Sodium carbonate is a reagent that commonly used to modify the pulp pH. However, the carbonate ions will not only change the pH, but can also increase nickel flotation selectivity over MgO minerals (Pietrobon et al., 1997). Sodium carbonate may have an influence on both the mineral surfaces and solution chemical species. The mechanism by which sodium carbonate improves nickel flotation recovery has not been elucidated to date. In this study, the effect of sodium carbonate on the flotation performance of a nickel ore was studied and the mechanism investigated.
Experimental
Materials and reagents
The serpentine (lizardite) used for the FTIR study was obtained from Donghai, Jiangsu Province, China. The mineral composition of the lizardite as determined by XRD was: lizardite 98%, chlorite 2%.
The ore sample used in this study is of high nickel grade (1.35% Ni). Approximately 96% of the nickel content is in the form of pentlandite and can be recovered by true flotation. The remaining 4% of nickel is distributed in non-sulphide minerals, and can be recovered only by entrainment or as composites with pentlandite. The ore contains 37% w/w MgO, distributed mainly in lizardite (46% w/w) and olivine (19% w/w), as determined by X-ray diffraction.
PAX (potassium amyl xanthate) and MIBC (methyl isobutyl carbinol) were used as collector and frothier respectively. All the reagents used in this study were of analytical grade.
Methods
Grinding
Ore samples were crushed to -2 mm, riffled into representative samples of 500 g, purged with nitrogen, and frozen during storage. For each flotation experiment, samples were ground in a mild steel rod mill to a P70 of 74 μm. Sodium carbonate was added at the grinding stage.
Flotation
The flotation tests were performed in a XFD-63 flotation cell (self-aeration) with a flotation volume of 1.5 L, using an agitation speed of 2800 r/min. The solid concentration in the flotation cell was 35% by weight. The pulp pH was maintained at 9 owing to the buffer effect of the minerals within the pulp. During the conditioning, collector (150 g/t) and frother (30 g/t) were added and conditioned for 5 minutes respectively to allow for collector and frother adsorption. After conditioning, flotation was started with the injection of air into the flotation cell. The air flow rate was maintained at 0.1 Nm3/h, monitored with a flow meter. Flotation was performed for 15 minutes and five concentrates were collected after cumulative times of 1, 3, 6, 10, and 15 minutes (Feng et al., 2012).
Sedimentation test
To study the effect of sodium carbonate on the settling behaviour of the pulp, sedimentation tests were performed. Ore samples were ground to a P70 of 74 μm. Sodium carbonate was added at the grinding stage when needed. 100 ml aliquots of ground pulp were transferred to a 100 ml measuring cylinder, and the height of the upper clear layer recorded at fixed times.
Sieving test
The floatability of pentlandite depends on the amount of slimes on its surface. To study the effect of sodium carbonate on the amount of slimes removed from the surfaces of coarse sulphide particles, sieving tests were performed. Pulps were sampled from the flotation cell before and after the addition of sodium carbonate and sieved to remove -75 μm particles. The particle size distribution of the +75 μm pulp samples was measured using a Malvern Master Sizer X (using tap water as a carrier for the measurements). The samples were measured after sonication (in a sonication bath attached to the Malvern Master Sizer X) for 4 minutes at the maximum sonication intensity (Chen et al., 1999a, 1999b). Portions of the +75 μm samples were treated by sonication and re-sieved at 75 μm and the -75 μm particles collected for XRD analysis.
FTIR study
In order to study the interaction of carbonate with lizardite, the IR (infrared) spectra of lizardite before and after sodium carbonate treatment were measured using a Nexus 670 series Fourier transform infrared spectrometer in the range 4000350 cm-1. For the analysis of pure sodium carbonate or lizardite, a 20 mg sample was added to 200 mg KBr, for a concentration of approximately 10 wt%. To test the infrared spectra of lizardite treated with sodium carbonate, 0.5 g samples of pure lizardite were individually suspended in 200 ml of sodium carbonate solution for 5 minutes. The pulps were then centrifuged and washed at least three times with distilled water and dried in a vacuum oven at 40°C. The infrared spectra were obtained as for the pure lizardite sample.
Results and discussion
The ore contained a large amount of lizardite slimes after grinding. The slimes interfered with the pentlandite flotation by adsorbing onto its surface. In the past, the concentrator used carboxymethyl cellulose to disperse and depress the slime. Sodium carbonate is a reagent that is commonly used to modify the pulp pH, and it can also be used to precipitate metal ions and disperse slime. To further improve the flotation recovery of pentlandite, sodium carbonate was considered as a dispersing agent on the basis of the original reagent regime.
The effect of sodium carbonate on the recovery and grade of the nickel ore is shown in Figure 1, and the effect of flotation time on the recovery with different sodium carbonate dosages is shown in Figure 2. The flotation results indicate that the addition of 5 kg/t sodium carbonate increased the nickel recovery from 82% to 90%, while the Ni grade did not change.


To study the mechanism by which sodium carbonate increased the flotation recovery of pentlandite, the effect of sodium carbonate on the settling behaviour of the nickel ore was investigated. The results (Figure 3) show that the addition of sodium carbonate reduced the settling velocity of the nickel ore, indicating that sodium carbonate has a dispersion effect on the pulp.

The effect of sodium carbonate on surface cleaning of the coarse particlea was investigated by the sieving tests and the results are shown in Figure 4. Pulps were sampled from the flotation cell before and after the addition of sodium carbonate and sieved at 75 μm to remove -75 μm particles. Ideally, no particles less than 75 μm should remain in the +75 μm fraction after sieving, unless those particles are adhering to the +75 μm coarse particles through slime coating. Thus, the change of size distribution of the fines (-75 μm fraction) in the +75 μm sample after the addition of sodium carbonate can be used to indicate the change in the amount of slimes on the coarse particle surfaces.

The results show that the amount of slimes removed from the surfaces of the coarse particles increased with increasing sodium carbonate dosage, and the coarse particle surfaces were free of particles less than 4 μm in size when 5 kg/t sodium carbonate was used. This shows that sodium carbonate is effective in assisting the removal of adhering slime particles from pentlandite surfaces.
The mineral composition of the slimes removed from large particles was investigated using XRD analysis. The results (Figure 5) suggest that the slimes attached to the surfaces of large particles were mainly lizardite, chlorite, and talc. The magnesium silicate gangue minerals are soft and more easily broken than the sulphide minerals in the grinding and conditioning processes. Thus the slimes consisted mainly of magnesium silicate gangue minerals.

The pH of the pulp after the addition of sodium carbonate is shown in Figure 6. The pulp pH remained essentially constant with the increase of sodium carbonate concentration. This result shows that sodium carbonate does not play a role in modifying the pulp pH.
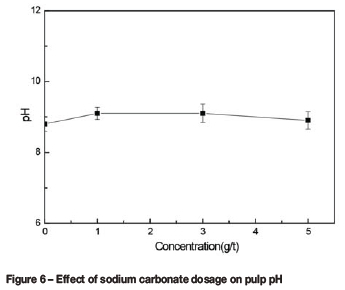
The pH regulators are divided in two main groups: reagents that produce hydroxyl (OH-) by dissociation and reagents which form OH- by hydrolysis. Sodium carbonate belongs to the latter group. Equations [1]-[3] show hydrolysis of carbonate. The ore contains 46% lizardite (w/w), which will consume carbonate; thus, there will not be sufficient carbonate ions left in the pulp to alter the pH.

In a previous study, it was demonstrated that sodium carbonate can effectively disperse lizardite and pyrite, improving the flotation performance of pyrite, which is depressed by lizardite (Feng and Luo, 2013). In order to study how the carbonate interacted with the lizardite surface, the IR (infrared) spectra were measured, and the results are shown in Figure 7. The IR spectrum of lizardite shows a peak at 3686.3 cm-1due to external Mg-OH stretching vibration, and the adsorption band of 984.6 cm-1is assigned to stretching vibration of Si-O. The peaks at 580 cm-1and 443.6 cm-1corresponding to deformation of the Mg-OH bond and shear vibration of Mg-OH, respectively. The IR spectrum of sodium carbonate has a strong adsorption peak at 1441.5 cm-1, and there also exists a peak at 1621.1 cm-1, both of which are attributed to the C-O asymmetric stretching vibration results.

After treatment with sodium carbonate, a new adsorption peak of 1423.8 cm-1appeared in the infrared spectrum of lizardite, which is the result of carbonate adsorbed on the surface of lizardite.
The particles finer than 5 μm in diameter are generally described as 'slimes'. The detrimental effect of slimes on flotation is encountered in many mineral systems. One of the reasons is related to their coating on valuable mineral particles. The dominant component in this nickel ore is lizardite, which is soft and easily broken during the grinding process to form slimes.
Surface charge is an important factor in controlling particle-particle interactions. The formation of slime coatings is directly related to the surface potentials of the sulphide minerals and lizardite particles. The lizardite has a positive potential at the pH range in which the flotation of nickel sulphide ore is performed; it is therefore likely that it will attach through electrostatic attraction to the negatively charged sulphide surface and form slime coatings. A coating of hydrophilic lizardite slimes will decrease the hydrophobicity of the sulphide particle and result in lower sulphide mineral recovery.
The sodium carbonate can interact with lizardite and change the surface charge to a negative value. The repulsive forces due to like charges are greater than the attractive van der Waals forces and some slimes are removed from the sulohide surface. The amount of slime removed is related to the dosage of sodium carbonate.
Conclusions
The valuable mineral of the nickel ore is pentlandite and the main gangue mineral is lizardite. The lizardite breaks readily during the grinding process and produces slimes, which are positively charged at the pH where flotation of nickel sulphide ore is performed. Thus the slime will adsorb onto the negatively charged pentlandite surface through electrostatic attraction and depress pentlandite flotation. Sodium carbonate interacts with slime particles and removes adhering slimes from pentlandite surfaces. The coarse particle surfaces were free of particles less than 4 μm in size at a sodium carbonate addition of 5 kg/t. This work has shown that carbonate can improve pentlandite flotation performance.
Acknowledgements
The authors acknowledge the support of the National Natural Science Foundation of China (Nos.51404109), and the National Basic Research Program of China (Nos. 2014CB643400).
References
Bremmell, K.E., Fornasiero, D., and Ralston, J. 2005. Pentlandite-lizardite interactions and implications for their separation by flotation. Colloids and Surfaces A - Physicochemical and Engineering Aspects, vol. 252, no. 2/3. pp. 207-212. [ Links ]
Chen, G., Grano, S., Sobieraj, S., and Ralston, J. 1999a. The effect of high intensity conditioning on the flotation of a nickel ore, Part 1: Size by size analysis. Minerals Engineering, vol. 12, no. 10. pp. 1185-1200. [ Links ]
Chen, G., Grano, S., Sobieraj, S., and Ralston, J. 1999b. The effect of high intensity conditioning on the flotation of a nickel, part 2: Mechanisms. Minerals Engineering, vol. 12, no. 11. pp. 1359-1373. [ Links ]
Edwards, G.R., Kipkie, W.B., and Agar, G.E. 1980. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite on pentlandite flotation. International Journal of Mineral Processing, vol. 7, no. 1. pp. 33-42. [ Links ]
Feng, B. and Luo, X. 2013. The solution chemistry of carbonate and implications for pyrite flotation. Minerals Engineering, vol. 53. pp. 181-183. [ Links ]
Feng, B., Feng, Q., Lu, Y., and Lv, P. 2012. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore. Minerals Engineering, vol. 39. pp. 48-50. [ Links ]
Kirjavainen, V. and Heiskanen, K. 2007. Some factors that affect beneficiation of sulphide nickel-copper ores. Minerals Engineering, vol. 20, no. 7. pp. 629-633. [ Links ]
Learmont, M.E. and Iwasaki, I. 1984. Effect of grinding media on galena flotation. Mineral and Metallurgical Processing, vol. 1. pp. 136-143. [ Links ]
Lu, Y.P., Zhang, M.Q., Feng, Q.M., Long, T., Ou, L.M., and Zhang, G.F. 2011. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Transactions of the Nonferrous Metals Society of China, vol. 21. pp. 208-213. [ Links ]
Pietrobon, M.C., Grano, S.R., Sobieraj, S., and Ralston, J. 1997. Recovery mechanisms for pentlandite and MgO-bearing gangue minerals in nickel ores from Western Australia. Minerals Engineering, vol. 10, no. 8. pp. 775-786. [ Links ]
Trahar, W.J. 1981. A rational interpretation of the role of particle size in flotation. International Journal of Mineral Processing, vol. 8. pp. 289-327. [ Links ]
Wellham, E.J., Elber, L., and Yan, D.S. 1992. The role of carboxy methyl cellulose in the flotation of a nickel sulphide transition ore. Minerals Engineering, vol. 5. pp. 381-395. [ Links ]
Paper received Apr. 2015
Revised paper received July 2015.














