Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.12 Johannesburg Dec. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n12a11
PAPERS - CHEMICAL ENGINEERING & METALLURGY AT WITS
Polyethersulphone-sodalite (PES-SOD) mixed-matrix membranes: Prospects for acid mine drainage (AMD) treatment
M.O. DaramolaI; B. SilindaI; S. MasondoI; O.O. OluwasinaI, II
ISchool of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Wits 2050, Johannesburg, South Africa
IIDepartment of Chemistry, Federal University of Technology, Akure, Nigeria
SYNOPSIS
This article presents the outcome of a preliminary investigation into the application of polyethersulphone (PES)-sodalite (SOD) mixed-matrix membranes for acid mine drainage (AMD) treatment. PES-SOD membranes loaded with different amounts of SOD particles were fabricated using the phase inversion method, and evaluated for AMD treatment. The morphology, phase purity, and surface properties of the SOD particles and the membrane were checked using scanning electron microscopy (SEM), X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy, respectively. In addition, the mechanical strength of the membranes was evaluated using a texture analyser. Separation performance (metal ion rejection) of the membranes and the effect of SOD loading on the membrane performance during AMD treatment were also studied. The cations in the AMD (feed stream) and the permeate stream were determined quantitatively using atomic absorption spectrophotometry (AAS). The results of the investigation reveal that mechanical strength (Young's modulus and tensile strength) of the membrane was enhanced at increasing SOD loading. In addition, the membrane flux increased at increasing SOD loadings and the selectivity of the membrane towards Mn2+, Pb2+, Cu2+, Al3+, and Mg2+also increased. The highest membrane rejection of 57.44% was recorded for Pb2+, and the membrane displayed a rejection of 6% towards Mn2+. All the PES-SOD membranes displayed better performance compared to an equivalent unloaded PES membrane. As far as we know, this is the first report on the application of PES-SOD mixed-matrix membranes to AMD treatment. However, optimization of the synthesis protocol and operational conditions is needed to improve the performance of the membrane.
Keywords: mixed matrix membranes, polyether sulphone, acid mine drainage, sodalite.
Introduction
Environmental pollution is better prevented than remediated, but most often prevention is difficult. The industrial revolution has had adverse consequences as well as benefits. For example, countries with past and current mining activities are facing challenges of environmental pollution due to acid mine drainage (AMD). The mining sector in South Africa is one of the main driving forces of the country's economy. Despite the positive benefits, the serious impact of mining activities on the environment resulting from AMD cannot be over-emphasized (Hughes and Gray 2013). Acid mine drainage has significant effects on environmental sustainability and water security (Evangelou, 1998).
AMD is produced by oxidation of sulphide minerals, mostly iron sulphides. This could be natural through the breaking down of sulphides by bacteria, as well as through anthropogenic activity, which is mining (Akcil and Koldas, 2006). Oxidation of sulphides produces sulphuric acid, which in turn leaches out a range of metals from rock and other metal-containing materials. This makes AMD potentially dangerous to the environment, since is not only highly acidic, but also contains high metal concentrations (Singh, 1987).
Research and development has been channelled towards source control and mitigation of AMD. Since oxygen and water are required for the formation of AMD, source control is targeted at preventing oxygen and/or water from contacting the sulphide-bearing rock so as to prevent oxidation reactions from producing AMD (Kuyucak 2002; Johnson and Hallberg, 2005; Luptakova et al., 2010; Egiebor and Oni, 2007). Preventing the formation and migration of AMD from its source is not easy. Thus, research activities are now focusing on collection, treatment, and discharge of treated AMD. Treatment techniques can be divided into two categories: active and passive treatments (Johnson and Hallberg 2005). This has been discussed extensively by many authors (Moses et al., 1987; Skousen et al., 1998; Johnson and Hallberg, 2005; Kalin et al., 2006; Egiebor and Oni 2007; Rotting et al., 2011). These days, various methods are employed for the treatment of AMD. Lime (Ca(OH)2 or limestone (CaCO3) treatment is carried out to precipitate the sulphate as gypsum and heavy metals as hydroxides (Lyew et al., 1994; Hedin et al., 1994; Dempsey and Jeon, 2001; Sibrell and Watten, 2003; Santomartino and Webb, 2007). This has a disadvantage because of the great quantities of gypsum sludge contaminated with metals that are produced, and the high operating costs for disposal (Johnson and Hallberg, 2005). Another method that is commonly used is two-step neutralization and ferrite formation, in which magnesium oxide or calcium carbonate is used for the first neutralization step to raise the pH to around 4.8 to produce low-solubility heavy metal hydroxide sludge (Igarashi et al., 2006; Herrera et al., 2007). The second stage employs sodium hydroxide to bring the pH to 8.5 to precipitate ferrous and ferric hydroxides together with the remaining heavy metals (Al-Zoubi et al., 2010). Biological treatment, which involves the use of sulphate-reducing bacteria, removes metals from AMD. This involves the use of various carbon-containing substances (such as manure, wood chips, food waste) to reduce sulphate to sulphide and form a metal sulphide precipitate (Tuttle et al., 1969; Wakao et al., 1979; Wildeman and Laudon, 1989; Ueki, 1991; Dvorak et al., 1992). The addition of the organic materials generates hydrogen sulphide which elevates the reaction temperature, which in turn decreases the effectiveness of the method (Dvorak et al., 1992; Barnes et al., 1992).
Furthermore, cation exchange processes have been proposed for the removal of toxic metals from AMD. However, the operating cost of the process is higher than the value of the metals recovered, and the exchange technology cannot cope well with the vast volumes of AMD discharge (Riveros, 2004; Al-Zoubi et al., 2010).
Recently, the application of membranes to treat AMD by selectively separating the heavy metals has been proposed and tested (Al-Zoubi et al., 2010; Jacangelo et al., 1997; Hilal et al., 2007; Escobar et al., 2000). A membrane is a barrier that selectively allows the desired molecule to permeate while undesirable molecules are retained (see Daramola et al., 2012, 2010 for detailed information on membranes and their classifications). Separation of mixtures by membranes can be carried out more efficiently and at lower energy consumption compared to distillation columns or absorbers (Ulbricht, 2006; Daramola et al., 2012). In addition, membrane technology is highly flexible, and the process can be adapted in response to changes in volumes and concentration of the mixture to be separated (Ulbricht, 2006). Furthermore, application of membrane systems in AMD treatment could reduce the usage of chemicals and sludge production, thereby making the treatment process environmentally benign. In the study by Al-Zoubi et al. (2010), three commercial membranes, namely nanofiltration (NF), ultrafiltration (UF), and reverse osmosis (RO) membranes, were tested for AMD treatment and the results yielded about 98% metal ions rejection. However, for effective treatment of AMD for safe disposal using the aforementioned membranes, two or three stages involving two or three of the membranes would be required, translating into additional capital and operating costs. To avoid this situation, a single-stage treatment of AMD using mixed matrix membranes is proposed in this article.
Mixed matrix membranes (MMMs) are composite membranes containing zeolite crystals within the matrix of the polymer membranes. The presence of crystals within the polymer chains improves separation performance, mechanical strength, and thermal stability of polymeric membranes. Advantages of MMMs over pure polymeric membranes include desirable mechanical properties, economical process-ability, unique structure of the dispersed inorganic phase, and good surface chemistry. However, the chemical structure of the inorganic fillers, type of inorganic fillers, and surface chemistry are mitigating factors to obtaining high-quality MMMs (Vu and Koros, 2003). In this study, synthesis and performance evaluation of poyethersulphone(PES)-sodalite mixed matrix membranes for AMD treatment are reported. Sodalites are zeolites possessing a framework with cubic symmetry structure, consisting of vertex linking of AlO4and SiO4 into four- and six-membered oxygen rings (Breck, 1974). Different types of sodalite can be synthesized for different applications because they can accommodate a wide range of cations as a result of framework flexibility (Breck, 1974). This could be exploited in the treatment of AMD, since AMD consists of dissolved substances (e.g. heavy metals) that are cationic or anionic in nature. Therefore, the sodalite particles could accommodate these substances during selective treatment of AMD.
In a recent study, the separation performance of supported sodalite/ceramic membrane for seawater desalination was reported and ultra-pure water was produced from seawater (Khajavi et al., 2010). Considering the outstanding performance of this membrane for water treatment (Khajavi et al., 2010, 2007, 2008), it is expected that using sodalite (SOD) as fillers in PES to fabricate PES-SOD mixed matrix membranes might result in a membrane with reasonable performance, in terms of water fluxes and selectivity, for AMD treatment. Against this background, the results of the preliminary investigation on the synthesis and application of PES-SOD mixed matrix membranes for acid mine drainage treatment are documented in this article.
Experimental
Chemicals
Solvent (N,N-dimethylacetamide, 97%), polyethersulphone (PES), sodium silicate, sodium aluminate, and sodium hydroxide were purchased from Sigma-Aldrich South Africa. Deionized water was prepared in-house. AMD samples were directly sourced from a small stream at the mine dumps in Dobsonville in Gauteng Province, South Africa. All chemicals were used as supplied without any further purification.
Synthesis of SOD crystals and PES-SOD membrane
Hydroxysodalite (SOD) crystals were prepared via hydrothermal synthesis using sodium metasilicate, sodium hydroxide pellets, anhydrous sodium aluminate, and deionized water. These materials were mixed together in a polytetrafluoroethylene (PTFE) bottle and stirred for 1 hour on a magnetic stirrer to yield a homogeneous mixture of molar composition ratio 5SiO2:Al2O3:50Na2O:1005H2O. Approximately 45 mL (or 48 g) of the vigorously mixed precursor solution was poured into a Teflon-lined stainless steel autoclave and subjected to hydrothermal synthesis at 413 K for 3.5 hours as described by Khajavi et al. (2010). At the end of the hydrothermal synthesis, the as-prepared SOD crystals were washed thoroughly with deionized water until the pH of the water was neutral. The washed crystals collected on filter paper were dried overnight at 373 K in an oven.
A homogeneous mixture for the fabrication of each membrane was prepared by measuring specific amounts of SOD crystals, polymer, and solvent with the ratio of SOD/PES to solvent in the mixture maintained at 1:9 throughout the syntheses. The amount of the PES was kept at 0.40 g throughout while the quantities of SOD particles and solvent were varied. Consequently, the weight percentages of the SOD crystals in the synthesized PES-SOD membranes were 5 wt.%, 10 wt.%, and 15 wt.%. Membranes were fabricated by hand-casting the homogeneous mixture on a glass plate using 'DR BLADE'. Reproducibility of synthesis was ensured by fabricating two batches under the same conditions, but at different times and locations. In addition, pure PES membrane (with 0 wt.% SOD) was synthesized for comparison. Figure 1 depicts the steps involved in the synthesis of the membranes.
Characterization of SOD crystals and PES-SOD membrane
The morphology and crystallinity of the synthesized SOD crystals were checked with scanning electron microscopy (SEM) using energy-dispersive X-ray spectroscopy (EDS) (Phillips XL 20), and X-ray diffractometry (XRd) (Bruker D8 advance X-ray diffractometer) using CoKa radiation (λ=0.179 nm) at a scan rate of 0.25 seconds per step and a step size of 0.02°, respectively. Further confirmation of the purity of the SOD crystals was obtained using Fourier transform infrared (FTIR) spectroscopy, conducted with a Bruker IFS spectrometer using KBr pellets as background.
Furthermore, the morphology of the fabricated mixed matrix membranes was examined using SEM.
Performance evaluation of PES-SOD membrane during AMD treatment
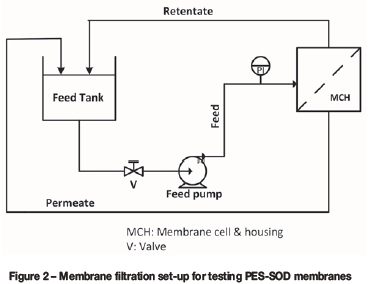
The performance of the membranes during AMD treatment was evaluated using a cross-filtration set-up depicted in Figure 2. Filtration tests were conducted at a feed temperature of 298 K and a pressure of 1.1 bar, using the membrane of effective permeation area 45 cm2. Atomic absorption spectrophotometry (AAS) was used to determine the concentration of various metal ions in the AMD before treatment (feed stream) and after treatment (permeate and retentate streams). The concentration of the metal ions in raw AMD as per the AAS analysis is summarized in Table I. The pH of the raw AMD obtained from the pH meter (model: HI 2550 pH/ORP and EC/TDS/NaCl meter) was 2.28. The membrane flux and the rejection (expressed in percentage) of the metal ions were obtained using Equation [1] and Equation [2]), respectively:

where Qp is the permeate mass flow rate (g/min), Jp is the permeate flux (g.cm-2.min-1), and A is the effective membrane area (cm2).

where Riis the percentage rejection of component i (%), and Cfand Cpare the concentrations of component i in the feed and permeate streams (mg/L), respectively.
Results and discussion
Synthesis and characterization of SOD crystals
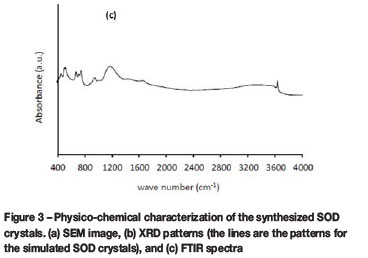
The morphology of the synthesized particles is shown in the SEM image in Figure 3(a). Figure 3(b) and Figure 3(c) confirm the crystallinity and the purity of the synthesized SOD crystals. The typical cubic shape of the SOD crystal is visible in Figure 3(a), confirming the formation of SOD crystals during the synthesis. Other morphological shapes such as nano-rod crystals were observed alongside the cubic SOD crystals in Figure 3(a). The same observation has been reported in the literature (Kundu et al., 2010). Figure 3(b) reveals the formation of pure SOD crystals when compared to standards developed by the Joint Committee on Powder Diffraction Standards (http://www.icdd.com), and the EDS analysis of the SOD crystals showed a Si/Al ratio of 1-1.5, indicating a SOD framework. The FTIR patterns depicted in Figure 3(c) confirm the appearance of all characteristic vibrational bands of SOD crystals. The strong broad band centred at approximately 1000 cm-icould be attributed to the asymmetric stretching vibration of T-O-T (T=Si, Al). The symmetric stretching vibration of T-O-T is vividly shown around 740 and 660 cm-1. The results obtained from the FTIR analysis of the SOD are consistent with the literature (Yao et al., 2006; Breck, 1974).
Characterization of the synthesized PES-SOD membranes
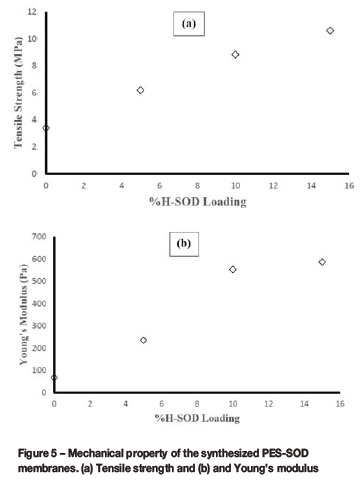
Figure 4 shows that SOD crystals were well distributed within the matrix of the polymer and that the ratio of the fractional free volume (FFV) of the polymer to the amount of sodalite particles within the polymer matrix decreased at increasing SOD loading. The observed difference between the loaded membranes and the unloaded polymeric membrane can be seen by comparing Figure 4(a) with Figures 4(b)-(d). The presence of embedded SOD is clear from Figure 4(b)-Figure 4(d), while there are no SOD particles in Figure 4(a). The presence of embedded SOD crystals in the PES confirms successful fabrication of a PES-SOD mixed matrix membrane. The results of the mechanical property evaluation using a TA.XT Plus texture analyser at ambient temperature are depicted in Figure 5. The tensile strength and the Young's modulus of the synthesized membranes increased with increasing SOD loading. This is consistent with the literature (Shen and Lua, 2012). The 15 wt.% loaded membrane displayed the largest tensile strength of 10.62 MPa, an increase of about 213% compared to that of the PES membrane (0 wt.% SOD loaded membrane). This observation further confirms the enhancement of the mechanical strength of polymer membranes with the addition of fillers, and the increase in strength with increasing filler loading (Wei et al., 2014). A slight increase in the Young's modulus for 15 wt.% SOD loaded membrane compared to those for 5 wt.% and 10 wt.% loaded membranes could be attributed to the ineffective dispersion method adopted in the synthesis of the membranes. At higher particle loadings, agglomeration of the particles may occur within the polymer matrix, causing a slight decrease in the Young's modulus (Wei et al., 2014; Shen and Lua, 2012; Yu et al., 2013). However, this could be avoided if an effective dispersion method is used for the synthesis of the membranes (Daramola et al., n.d.).
Performance evaluation of PES-SOD membrane for AMD treatment
The AMD sample contained high concentrations of iron, aluminium, and magnesium ions, and moderate concentrations of copper (Cu2+), zinc (Zn2+), lead (Pb2+), and manganese (Mn2+) ions (see Table I). The high Fe3+ concentration could be attributed to continuous oxidation of Fe2+ to (Fe3+) as a result of a pH lower than 3 (Stumm and Morgan, 1996). The AMD sample was considered hard since the concentration of magnesium ions (848.78 mg/L) was greater than 120 mg/L (Al-Zoubi, et al., 2010).
The performance of each membrane was evaluated at a pump speed of 12 revolutions per second. The concentrations of metal ions and the pH of the permeate sample from each membrane were determined using AAS and a pH meter, respectively. Figure 6 shows the pH results of the permeate sample for each membrane after the treatment. A small increase in the pH from 2.28 for the feed sample to 2.50 (about 9.65% increase) was observed for the permeate samples from the four membranes after the treatment. The small change in the pH could be attributed to a smaller rejection of Fe3+during the AMD treatment, because the presence of Fe3+ lowers pH (Stumm and Morgan, 1996), and a higher rejection of Fe3+ is expected to increase the pH. In addition, an increase in the pH was observed at increasing SOD loading, implying that the performance of the PES-SOD membranes was enhanced with greater SOD loading.
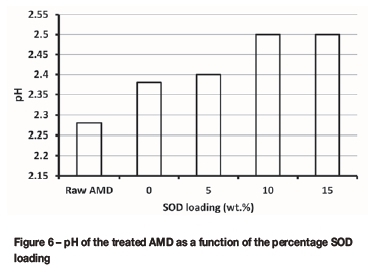
Figure 7 shows that the permeate flux of the membranes increased at increasing SOD loading (0 wt.% to 15 wt.% loading), reached a maximum of 1.85 g.cm-2.min-1at 10 wt.% loading, and then decreased to 1.43 g.cm-2.min-1at 15 wt.% loading. The observed increase in the membrane flux of the PES-SOD membranes when compared to that of the PES (0 wt.% loading) membrane could be attributed to the presence of the SOD crystals and the hydrophilic nature of the crystals. In addition, the kinetic diameter of water molecules (2.65 A) and the average cage dimension of the SOD particle (2.6 A) are comparable, implying that the SOD particles provide additional permeation channels for the water molecules in the PES-SOD when compared to the membrane flux of the unloaded PES membrane.
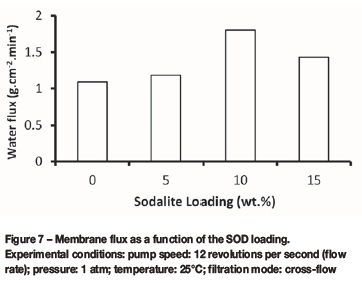
The water transport mechanism in porous hydrophilic membranes has been described by a sorption-diffusion mechanism and preferential sorption capillary flow (PSCF) model (Sourirajan, 1963). According to the sorption-diffusion mechanism, water molecules preferentially adsorb to the hydrophilic membrane surface, diffuse through the membrane pores, and desorb from the membrane. The PSCF model describes transport of water through such membranes as occurring by hydrogen bonding, during which water molecules are attracted by the hydrogen bonds within the membrane and permeate through the membrane pores. Indeed, the separation efficiency of membranes depends on their surface properties (hydrophilicity or hydrophobicity) and pore dimensions (with respect to the molecular size of the adsorbates). The permeation of water molecules through the synthesized PES-SOD membranes is therefore expected to be governed by the PSCF model because cages of SOD crystals are occluded with water, indicating that the transport of water molecules through the crystals could occur by hydrogen bonding. On the other hand, the observed decrease in the membrane flux at higher (15 wt.%) SOD loading could be attributed to membrane fouling due to the retention of increased numbers of particles from the AMD. However, it is noteworthy to mention that the AMD sample was pre-treated to remove the suspended particles before the filtration tests. Interestingly, the 15 wt.% loaded membrane did not lose its selectivity, indicating few or no defects in the membrane.
Furthermore, the performance of the PES-SOD membranes was evaluated using selectivity of the membrane to the metal ions. Figure 8 depicts the percentage rejection of each metal ion by the membranes as a function of the SOD loading. Lead (Pb2+) was the most rejected of all the metal ions with a maximum rejection of 57.44%. This is expected because the kinetic diameter of Pb2+ is 2.66 Å (Salam et al., 2012) and the average cage dimension of the SOD particles is 2.6 A (Breytenbach et al., 2007). Therefore, more Pb2+was retained by the membrane, thereby resulting in high selectivity of the membrane for Pb2+. Rejection of magnesium (Mg2+) increased from about 20% to about 50% with increasing SOD loading. However, to soften the AMD, a higher rejection of Mg2+is required. Mn2+was the least rejected metal ion with a rejection from 1% to 6%. The maximum percentage Cu2+ rejection of 17.6% displayed by the membranes was not very encouraging. This performance could be attributed to the smaller kinetic size of the Cu2+ (about 1.44 Å) compared to the average pore dimension of SOD crystals (Breytenbach et al., 2007). Cu2+ could be easily filtered through the embedded SOD particles. It is evident from the results that none of the metals ions in the AMD sample was well rejected with the synthesized membranes. However, modifications to the membranes by functionalizing the SOD particles and optimizing the synthesis protocol could dramatically enhance the performance of the PES-SOD membranes. Additionally, process conditions during the treatment of AMD with the membranes could be optimized to enhance the flux and selectivity of the membranes.
Conclusion
Results of a preliminary investigation of the application of PES-SOD mixed matrix membranes to AMD treatment are reported for the first time. The synthesis protocol adopted resulted in the fabrication of selective and reproducible PES-SOD mixed matrix membranes. Three different PES-SOD mixed matrix membranes were fabricated with different SOD loadings, as well as a pure polymeric membrane for comparison. The experimental results showed that the performance of a pure polymeric membrane (selectivity and flux) is enhanced by loading SOD crystals within the matrix of the PES polymer. Results of the performance evaluation of the membranes for AMD treatment revealed the best water flux at 10 wt.% SOD loading and a pump speed of 12 revolutions per second. The PES-SOD loaded with 15 wt.% SOD crystals displayed the best selectivity towards Pb2+ (57.44% rejection). The results from this study have shown the potential for application of PES-SOD membranes in the treatment of AMD. However, optimization of the synthesis protocol for the fabrication of the membrane and process conditions might be required to enhance the performance of the membranes for AMD treatment. Nevertheless, the results documented in this article could pave the way for further research and development in this area.
Acknowledgements
M.O.D. acknowledges Professor F. Kapteijn and Professor J. Gascon of the Delft University of Technology, The Netherlands, for the opportunity to gain from their wealth of experience in the membrane field. The authors also acknowledge Dr D. Nkazi for assisting with the collection of the AMD sample. This work emanated from the results of the 4th year BSc (Eng) chemical engineering research project conducted by BS and SM.
References
Akcil, A. and Koldas, S. 2006. Acid mine drainage (AMD): causes, treatment and case studies. Journal of Cleaner Production, vol. 14. pp. 1139-1145. [ Links ]
Al-Zoubi, H., Rieger, A., Steinberger, P., Pelz, W., Haseneder, R., and Härtel, G. 2010. Nanofiltration of acid mine drainage. Desalination and Water Treatment, vol. 21. pp. 148-161. [ Links ]
Al-Zoubi, H., Steinberger, P., Pelz, W., Haseneder, R., and Hartel, G. 2010. Optimisation study for treatment of acid mine drainage using membrane technology. Separation Science and Technology, vol. 45, no. 14. pp. 2004-2016. [ Links ]
Barnes, L., Janssen, F., Scheeren, P. Versteegh, J., and Koch, R. 1992. Simultaneous microbial removal of sulfate and heavy metals from waste water. Transactions of the Institution of Mining and Metallurgy, Section C, vol. 101. pp. 183-189. [ Links ]
Breck, D.W. 1974. Zeolite Molecular Sieves: Structure, Chemistry and Use. Wiley, New York. [ Links ]
Breytenbach, J.C., Jaco Zaha, J., Krieg, H.M., and Van Niekerk, A. 2007. Direct crystallisation of a hydroxy sodalite membrane without seeding using a conventional oven. Journal of Membrane Science, vol. 300. pp. 156-164. [ Links ]
Daramola, M.O., Aransiola, E.F., and Ojumu, T.V. 2012. Potential applications of zeolite membranes in reaction coupling separation processes. Materials, vol. 5, no. 11. pp. 2101-2136. [ Links ]
Daramola, M.O., Burger, A.J., Pera-Titus, M., Giroir-Fendler, A., Miachon, S., Dalmon, J.A., and Lorenzen, L. 2010. Separation and isomerization of xylenes using zeolite membranes: a short overview. Asia-Pacific Journal of Chemical Engineering, vol. 5, no. 6. pp. 815-837 [ Links ]
Daramola, M.O., Hlanyane, P., and Iyuke, S.E. Not dated. Effect of CNT dispersion method on the quality and performance of carbon nanotube/polysulfone composite membranes during oil-water mixture. Separation Science and Technology (submitted). [ Links ]
Dempsey, B. and Jeon, B. 2001. Characteristics of sludge produced from passive treatment of mine drainage. Geochemistry Exploration Environment Analysis, vol. 1. pp. 89-94. [ Links ]
Dvorak, D., Hedin. R., Edenborn, H., and McIntire, P. 1992. Treatment of metal-contaminated water using bacterial sulfate reduction: results from pilot-scale reactors. Biotechnology and Bioengineering, vol. 40, no. 5. pp. 609-616. [ Links ]
Egiebor, N.O. and Oni, B. 2007. Acid rock drainage formation and treatment: a review. Asia-Pacific Journal of Chemical Engineering, vol. 2. pp. 47-62. [ Links ]
Escobar, I., Hong, S., and Randall, A. 2000. Removal of assimilable and biodegradable dissolved organic carbon by reverse osmosis and nanofil-tration membranes. Journal of Membrane Science, vol. 175, no. 1. pp. 1-17. [ Links ]
Evangelou V.P. 1998. Pyrite chemistry: the key for abatement of acid mine drainage in acidic mining lakes. Acid Mine Drainage, Limnology and Reclamation. Geller, A. Klapper, H., and Solomons, W. (eds). Springer, Berlin. pp. 197-222. [ Links ]
Hedin, R., Watzlaf, G., and Nairn, R. 1994. Passive treatment of acid mine drainage with limestone. Journal of Environmental Quality, vol. 23, no. 6. pp. 1338-1345. [ Links ]
Herrera, S., Uchiyama, H., Igarashi, T., Asakura, K., Ochi, Y., Ishizuka, F., and KAWADA, S. 2007. Acid mine drainage treatment through a two-step neutralization ferrite-formation process in northern Japan: Physical and chemical characterization of the sludge. Minerals Engineering, vol. 20. pp. 1309. [ Links ]
Hilal, N., Al-Zoubi, H., Darwish, N., and Mohammed, A. 2007. Performance of nanofiltration membranes in the treatment of synthetic and real seawater. Separation Science and Technology, vol. 42. pp. 493. [ Links ]
Hughes, T.A. and Gray, N.F. 2013. Co-treatment of acid mine drainage with municipal wastewater: performance evaluation. Environmental Science and Pollution Research, vol. 20, no. 11. pp. 7863-7877. [ Links ]
Igarashi, T., Asakura, K., Yoshida, T., Miyamae, H., Iyatomi, N., and Hashimoto, K. 2006. Ferrite formation using precipitate in the treatment of acid mine drainage for reducing its volume. Proceedings of the 5th International Congress on Environmental Geotechnics, Cardiff, Wales, UK. pp. 909-916. [ Links ]
Jacangelo, J., Trussell, R., and Watson, M. 1997. Role of membrane technology in drinking water treatment in the united States. Desalination, vol. 113. pp. 119-127. [ Links ]
Johnson, D.B. and Hallberg, K.B. 2005. Acid mine drainage remediation options: a review. Science of the Total Environment, vol. 338. pp. 3-14. [ Links ]
Kalin, M., Fyson, A., and Wheeler, W.N. 2006. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Science of the Total Environment, vol. 366. pp. 395-408. [ Links ]
Khajavi, S., Jansen, J.C., and Kapteijn, F. 2010. Production of ultra-pure water by desalination of seawater using a hydroxy sodalite membrane. Journal of Membrane Science, vol. 356. pp. 52-57. [ Links ]
Khajavi, S., Kapteijn, F. and Jansen, J.C. 2007. Synthesis of thin defect-free hydroxy sodalite membranes: new candidate for activated water permeation. Journal of Membrane Science, vol. 299. pp. 63-72. [ Links ]
Khajavi,S., Kapteijn, F., and Jansen, J.C. 2008. Application of hydroxy sodalite films as novel water selective membranes. Journal of Membrane Science, vol. 326. pp. 153-160. [ Links ]
Kundu, D., Dey, B., Naskar, M.K., and Chatterjee, M. 2010. Emulsion-derived urchin-shaped hydroxy sodalite particles. Material Letters, vol. 64. pp. 1630-1633. [ Links ]
Kuyucak, N. 2002. Acid mine drainage prevention and control options. CIM Bulletin, vol. 95, no. 1060. pp. 96-102 [ Links ]
Luptakova, A., Balintova, M., Jencarova, J., Macingova, E., and Prascakova, M. 2010. Metals recovery from acid mine drainage. Nova Biotechnologica, vol. 10, no. 1. pp. 23-32. [ Links ]
Lyew, D., Knowles, R., and Sheppard, J. 1994. The biological treatment of acid mine drainage under continuous flow conditions in a reactor. Transactions of the Institute of Chemical Engineers, vol. 72 (B). pp. 42-47. [ Links ]
Moses, C.O., Nordstrom, D.K., Herman, J.S., and Mills, A.L. 1987. Aqueous pyrite oxidation by dissolved oxygen and ferric iron, Geochimica et Cosmochimica Acta, vol. 1. pp. 1561-1571. [ Links ]
Riveros, P. 2004. The extraction of Fe (III) using cation-exchange carboxylic resins. Hydrometallurgy, vol. 72, no. 3-4. pp. 279-290. [ Links ]
Rotting, T.S., Nieto, J.M., and Ayora, C. 2011. Long term remediation of highly polluted acid mine drainage: a sustainable approach to restore the environmental quality of the odiel river basin. Environmental Pollution, vol. 159. pp. 3613-3619. doi: 10.1016/j.envpol.2011.08.003 [ Links ]
Salam, M.A., Al-Zhrani, G., and Kosa, S.A. 2012. Simultaneous removal of copper(II), lead(II), zinc(II) and cadmium(II) from aqueous solutions by multi-walled carbon nanotubes. Comptes Rendus Chimie, vol. 15. pp. 398-408. [ Links ]
Santomartino, S. and Webb, J. 2007. Estimating the longevity of limestone drains in treating acid mine drainage containing high concentrations of iron. Applied Geochemistry, vol. 22, no. 11. pp. 2344-2361. [ Links ]
Shen, Y. and Lua, A.C. 2012. Preparation and characterization of mixed matrix membranes based on poly(vinylidene fluoride) and zeolite 4A for gas separation. Polymer Engineering and Science, vol. 52, no. 10. pp. 2106-2113. [ Links ]
Skousen, J., Rose, A., Geidel, G., Foreman, J., Evans, R., and Hellier, W. 1998. Handbook of Technologies for Avoidance and Remediation of Acid Mine Drainage. National Mine Land Reclamation Centre, West Virginia university, West virginia. [ Links ]
Sibrell, P. and Watten, B.J. 2003. Evaluation of sludge produced by limestone neutralization of AMD at the Friendship Hill National Historic Site. Proceedings of the 20th Annual Meeting of the American Society for Mining and Reclamation, Billings, Montana. pp. 1151-1169. [ Links ]
Singh, G. 1987. Mine water quality deterioration due to acid mine drainage. International Journal of Mine Water, vol. 6, no. 1. pp. 49-61. [ Links ]
Sourirajan, S. 1963. The mechanism of demineralization of aqueous sodium chloride solutions by flow, under pressure, through porous membranes. Industrial Engineering and Chemistry Fundamentals, vol. 2. pp. 51-55. [ Links ]
Stumm, W. and Morgan, J. J. 1996. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Water. Wiley, New York. [ Links ]
Tuttle, J., Dugan, P., Macmillan C., and Randle, C. 1969. Microbial dissimi-latory sulfur cycle in acid mine water. Journal of Bacteriology, vol. 97, no. 2. pp. 594-602. [ Links ]
Ueki, K., Ueki, A., Itoh, K., Tanaka, T., and Satoh, A. 1991. Removal of sulfate and heavy metals from acid mine water by anaerobic treatment with cattle waste: effects of heavy metals on sulfate reduction. Journal of Environmental Science and Health A, vol. 26, no. 8. pp. 1471-1489. [ Links ]
Ulbricht, M. 2006. Advanced functional polymer membranes. Polymer, vol. 47. pp. 2217-2262. [ Links ]
Vu, D.Q., Koros, W.J., and Miller, S.J. 2003. Mixed matrix membranes using carbon molecular sieves II. Modeling permeation behaviors. Journal of Membrane Science, vol. 211. pp. 311-334. [ Links ]
Wakao, N., Takahashi, T., Sakurai, Y., and Shiota, H. 1979. A treatment of acid mine water using sulfate-reducing bacteria. Journal of Fermentation Technology, vol. 57, no. 5. pp. 445-452. [ Links ]
Wei, L., Qiong, W., Xin, Z., Zhanhua, H., Jun, C., Jian, L., and Shouxin, L. 2014. Enhanced thermal and mechanical properties of PVA composites formed with filamentous nanocellulose fibrils. Carbohydrate Polymers, vol. 113. pp. 403-410. [ Links ]
Wildeman, T. and Laudon, L. 1989. The use of wetlands for treatment of environmental problems in mining: non-coal mining applications. Proceedings of the International Conference on Constructed Wetlands for Wastewater Treatment. Hammer, D.H. (ed.). Lewis Publishing, Ann Arbor, MI. pp. 221-231. [ Links ]
Yao, J., Wang, H., Ratinac, K.R., and Ringer, S.P. 2006. Formation of colloidal hydroxy sodalite nanocrystals by direct transformation of silicalite-1 nanocrystals, Chemistry Materials, vol. 18. pp. 1394-1396. [ Links ]
Yu, L., Zhang, Y., Zhang, B., Liu, J., Haoqin, Z., and Song, C. 2013. Preparation and characterization of HPEI-GO/PES ultrafiltration membrane with antifouling and antibacterial properties. Journal f Membrane Science, vol. 447. pp. 452-462. [ Links ]
Paper received May 2015
Revised paper received Aug. 2015