Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.12 Johannesburg Dec. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n12a5
PAPERS - CHEMICAL ENGINEERING & METALLURGY AT WITS
Synthesis of sodium silicate from South African coal fly ash and its use as an extender in oil well cement applications
T. Kaduku; M.O. Daramola; F.O. Obazu; S.E. Iyuke
School of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, Wits 2050, Johannesburg, South Africa
SYNOPSIS
In this work, the use of sodium silicate derived from South African coal fly ash (CFA) in oil well cement (OWC) applications is reported. Silica (SiO2) was extracted from the CFA and used to synthesize CFA-derived sodium silicate (CFA-Na2SiO3), a typical OWC slurry extender. The physico-chemical properties of the CFA-Na2SiO3were compared to those of a commercial sodium silicate (com-Na2SiO3) using scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared (FTIR) spectroscopy. OWC slurries with varying proportions of cement, distilled water, and 2% CaCl2 by weight of water (BWOW) were prepared and extended using the CFA-Na2SiO3and the com-Na2SiO3at compositions ranging from 0.25-2.5% by weight of cement (BWOC). Rheological properties of the slurries were evaluated using American Petroleum Institute procedures and compared. The physico-chemical properties of the CFA-Na2SiO3are consistent with those of com-Na2SiO3, indicating the purity of the CFA-Na2SiO3. A comparative study of the OWC slurries indicated that the slurries extended with CFA-Na2SiO3have slightly lower densities, lower viscosities, and higher compressive strength than those extended with com-Na2SiO3. This indicates that CFA-Na2SiO3slurries would be easier to pump and preferable where early strength development is critical. This report could be instrumental in providing a way for the beneficiation of South African CFA in the petroleum, oil, and gas industry.
Keywords: oil well cementing, coal fly ash, sodium silicate, compressive strength.
Introduction
During the oil and gas well cementing process, some wells often lose circulation zones, and are therefore prone to formation breakage. Often, low-density slurries are required to overcome these problems, and extenders, which help to reduce the weight of the slurry, are utilized (Salim and Amani 2013; Ahmaruzzaman 2010; Shahriar 2011). The commonly used extenders are water extenders such as bentonite and sodium silicate, which allow addition of water to the slurry; and low-density aggregates such as microspheres and pozzolans, which have densities lower than that of Portland cement (3.15 g.cm-3) (Nelson et al., 1990). These extenders reduce the density of the slurry, resulting in a reduction of the hydrostatic pressure during cementing (Nelson et al., 1990). Extenders also increase slurry yield by replacing a substantial amount of the cement required to complete a given task, thereby reducing the expenditure.
One of the most commonly used water extenders for oil well cement (OWC) is sodium silicate (Na2SiO3). It has been reported that Na2SiO3 as a water extender is five times more effective than bentonite (Nelson et al. 1990). Unlike pozzolanic extenders such as fly ash, Na2SiO3 is highly reactive with OWC (Joel and Ujile, 2009). Na2SiO3 reacts with the Ca2+ions from the lime in the OWC or calcium chloride to produce additional calcium-silica-hydrate (C-S-H) gel (Nelson et al., 1990). The gel structure provides sufficient viscosity to allow the use of large quantities of mix water without excessive free water separation (Nelson et al., 1990). The further C-S-H formation also results in a reduction in thickening time, hence the accelerating effect of Na2SiO3 (Joel and Ujile, 2009). A low concentration of Na2SiO3is required for a high yield as compared to other extenders such as bentonite and raw coal fly ash, making it a preferred additive for OWC (Joel and Ujile 2009). While fly ash is added in concentrations of up to 50% by weight of cement (BWOC) and bentonite up to 20% BWOC, Na2SiO3additions range from 0.2% to 3.0% BWOC (Nelson et al., 1990). The accelerating effect of Na2SiO3, however, limits its application at lower temperatures, typically at less than 52°C bottom hole circulating temperature (BHCT) (Nelson et al., 1990; Joel and Ujile 2009). However, it can be used at higher temperatures with the addition of a retarder, although in the presence of a retarder, the effectiveness of Na2SiO3as an extender is reduced because of the inhibition of C-S-H formation (Nelson et al., 1990; Joel and Ujile, 2009).
At industrial scale, commercial sodium silicate is traditionally manufactured by calcination of sodium carbonate (Na2CO3) and SiO2 at a temperature range of 1400-1500°C in furnaces (Folleto et al., 2006). Although the raw materials are cheap, the process is not cost-effective due to its high energy consumption and maintenance cost (Folleto et al., 2006). The process also emits dust, nitrogen, and sulphur oxides, contributing to air pollution. Alternatively, Na2SiO3 can be produced by the reaction of SiO2 with NaOH solution in an autoclave, at high temperature and pressure (McDaniel et al., 1961). In light of the information at hand, it is necessary to carry out an investigation on alternative methods, which are less energy intensive, for producing Na2SiO3 for use as an additive to OWC. In addition, the SiO2, one of the raw materials required in the aforementioned alternative preparation method of Na2SiO3, can be obtained from coal fly ash (CFA).
CFA is an inorganic powder that is produced during the combustion of coal (Ahmaruzzaman, 2010). Several studies have been carried out and reported on the beneficiation of CFA (Iyer and Scott, 2001; Ahmaruzzaman, 2010; Taylor, 1990; Kamarudin et al., 2009; Park et al., 2012). Beneficiation of fly ash from other materials such as rice husks, bagasse, and corn cobs has also been reported (Aigbodion et al., 2010; Foleto et al., 2006; Okoronkwo et al., 2013). The use of CFA in OWC has been reported (Shahriar, 2011), but composition of the CFA differs from country to country and this could affect its suitability as additive in OWC operations. Furthermore, the use of raw CFA results in slower strength gain and longer setting times, thereby resulting in low early-age strength and delays in the well completion process (Bazzar et al., 2013). In South Africa, about 25 Mt of CFA is produced annually and its disposal constitutes a huge environmental problem. In this study, SiO2was extracted from CFA and used as starting material to synthesize Na2SiO3. The potential use of the synthesized CFA-derived sodium silicate as an OWC extender was evaluated and compared with that of commercial sodium silicate (com- Na2SiO3).
Materials and methods
Materials
Class G cement was obtained from Dyckerhoff, Germany, and CFA from a power station in South Africa. Demineralized water was prepared in-house. Hydrochloric acid solution (37%), sodium hydroxide pellets (98% purity), calcium chloride (99.99% purity), and commercial sodium metasilicate (95%) were purchased from Sigma Aldrich and used as delivered without any modification or purification.
Methods
CFA sample preparation and characterization
Small quantities of CFA were scooped randomly from different points and then mixed together to make the representative sample. The CFA samples were characterized using X-ray diffraction (XRD: Brucker D2 X-ray diffraction machine), X-ray fluorescence (XRF: PANalytical AXIOS X-ray fluorescence spectrometer), scanning electron microscopy (SEM: Zeiss Sigma VP field emission scanning electron microscope), and thermogravimetric analysis (TGA: TGA DSC STA 600 with Pyris software). The pH of CFA (slurried in water) was measured using a Metrohm 744 pH meter, and the particle size distribution of the CFA was obtained using a Malvern Mastersizer 2000.
Extraction of Si02 and preparation of Na2Si03 from CFA
The metal oxides such as Al2O3and CaO were removed from CFA by acid refluxing using 3 M HCl at 100°C for 6 hours as described by Tang et al. (2012). The solid product, SiO2, was filtered out and purified by successive washings with demineralized water. The wet SiO2 was dried in an oven at 200°C for 2 hours to obtain an amorphous silica powder which was then analysed using SEM-EDX, XRD, and Fourier transform infrared spectroscopy (FTIR: Bruker Tensor 27 Fourier transform infrared spectrometer). 60 g of the amorphous silica was reacted with 80 g sodium hydroxide pellets in 100 ml distilled water in a Pyrex flat-bottomed flask at 80°C and atmospheric pressure to produce a colourless viscous solution. The solution was then poured into a crucible and calcined at 300°C for 3 hours to produce a white solid (CFA-Na2SiO3) which was crushed to a powder using a mortar and pestle. The product was then subjected to SEM-EDX, XRD, and FTIR analyses.
Preparation and characterization of cement slurries
Slurries containing varying amounts of cement, distilled water, 2% calcium chloride (CaCl2) by weight of water (BWOW), com-Na2SiO3, and CFA-Na2SiO3 were prepared and their densities, rheology, and thickening times evaluated. A Chandler Ametek constant speed mixer (Model 30-60) was used for mixing and the slurries were pre-conditioned using a Chandler Ametek Atmospheric Consistometer (Model 1200) prior to the rheology tests. The rheology tests were conducted using a Chandler Ametek automated viscometer (Model 3530) and a Chandler Ametek pressurized mud balance was used to determine the density of the slurries. A Chandler Ametek twin cell ultrasonic cement analyser (UCA) (Model 4262) was used to determine the development of compressive strength of the slurries. All the tests on the cement slurries were carried out according to the specification for materials and testing for well cements (American Petroleum Institute Specification 10A, 2002). The compositions of the slurries are presented in Table I.
Results and discussion
CFA sample preparation and characterization
Figure 1 shows the XRD patterns for the CFA. The patterns are consistent with those reported for previously studied South African CFAs (Ayanda et al., 2012; Mainganye et al., 2013; Ikotun et al., 2014). Table II shows the XRF analysis of the CFA together with results from reports in the literature (Ayanda et al., 2012; Mainganye et al., 2013). The major components of the CFA are silica, alumina, iron oxide, calcium oxide, and carbon (inferred from the loss on ignition (LOI) test). The CFA is of class F (ASTM C618, 2012). The metal oxide contents decreased in the order SiO2> Al2O3> Fe2O3> CaO> MgO> K2O> Na2O> TiO2. This is consistent with previous reports on South African CFA (Ayanda et al., 2012; Mainganye et al., 2013; Ikotun et al., 2014). Interestingly, the CFA contained about 58% SiO2, which is one of the required materials for the synthesis of Na2SiO3.

The morphology of the CFA is depicted in the SEM micrograph in Figure 2. The shapes of the CFA particles are determined by the exposure conditions (time and temperature) in the combustion chamber (Fisher et al., 1978). As seen in Figure 2, most of the particles are spherical, especially in the finer fractions. A similar observation has been reported for other South African CFAs (Ayanda et al., 2012; Mainganye et al., 2013). The particles are a mixture of opaque and non-opaque spheres. The opaque spheres are predominantly iron oxides and some silicates, while the non-opaque spheres are mainly silicates (Fisher et al., 1978). Previous studies have shown that fly ash is made up of, in some cases, smaller particles (<< 1 μm) which are attached to the surface of larger particles, hollow spheres (cenospheres), and some spheres containing other spheres (plerospheres) (Mainganye et al., 2013). In addition, the SEM micrograph shows the presence of some non-spherical particles. These amorphous particles arise mainly from incomplete combustion of coal components (Fisher et al., 1978). Furthermore, the TGA shows that the CFA contains about 0.2% moisture, 1.6% volatile matter, and 1.7% fixed carbon. The particle size ranges from 0.32-112 μm. These results are also in agreement with previous studies (Ayanda et al., 2012 ; Ikotun et al., 2014). A rise in pH from 7 to 10.7 was observed when the CFA was mixed with de-ionized water over a period of 5 hours. This could be attributed to the dissolution of compounds such as CaO in the CFA. This observation is in good agreement with previous reports (Ayanda et al., 2012).

Extraction of SiO2, preparation and characterization of Na2Si03 from CFA
The morphologies of the extracted SiO2, synthesized CFA-Na2SiO3, and com-Na2SiO3were characterized by SEM. Figure 3 shows the SEM images of the extracted SiO2. Similar images for precipitated SiO2have been reported in the literature (Music et al., 2011). The elemental compositions obtained using energy dispersivee X-ray spectroscopy (EDS) (not shown in this manuscript) indicated the presence of Si and O, confirming the presence of SiO2. The XRD pattern in Figure 4 shows the characteristic slope and pattern for amorphous SiO2, consisting of a broad band with a peak which indicates that the substance is amorphous and contains pure SiO2 (Saikia et al., 2008; Essien et al., 2011; Music et al., 2011; Okoronwo et al., 2013). Similar patterns for amorphous silica have been recorded in the literature (Saikia et al., 2008; Okoronwo et al., 2013). The two sharp peaks in the pattern are due to the presence of quartz, corroborating the results obtained from EDS. The FTIR spectrum of the SiO2is depicted in Figure 5. The bands of absorption at 1199 cm-1, 964 cm-1, and 682 cm-1can be attributed to the absorption peaks characteristic of SiO2(Ying-Mei et al., 2010). The absorption peak at 1199 cm-1 corresponds to the asymmetrical stretching vibration of Si-O (Ying-Mei et al., 2010). In addition, the absorption peaks at 964 cm-1and 682 cm-1correspond to the symmetrical stretching vibrations of Si-O groups on the surface of the amorphous solid (Ying-Mei et al., 2010; Essien et al., 2011). The stretch between 1500 cm-1 and 2000 cm-1 can be attributed to the presence of the Si-OH and bending vibration absorption of the O-H bond of physically adsorbed water, respectively (Music et al., 2011).



The XRD patterns for CFA-Na2SiO3 and com-Na2SiO3 are shown in Figure 6. The two patterns are similar, although a small difference in the intensities of some of the peaks was observed. Curve fitting showed that there is a slight shift in the peaks of CFA-Na2SiO3. The shift in the peaks could be attributed to the small quantity of the sample used in the analysis. Figure 7 depicts the SEM images for CFA-Na2SiO3 and com-Na2SiO3. The morphology of the CFA-Na2SiO3 is totally different from that of com-Na2SiO3. However, the EDS results showed the same elemental components with different compositions. Figure 8 shows the FTIR spectra for the com-Na2SiO3 and CFA-Na2SiO3. The FTIR analysis of the two samples indicates that there is no observable chemical difference between the samples. Both samples show absorption bands at 2340 cm-1, 1160 cm-1, 1125 cm-1, 980 cm-1, and 715 cm-1that characterize the presence of sodium metasilicate (Miller and Wilkins, 1952). The stretch between 1500 cm-1 and 2000 cm-1 could be attributed to the presence of Si-OH and bending vibration absorption of the OH bond (Ying-Mei et al., 2010).



Preparation and characterization ofcement slurries
The compositions and the densities of the slurries prepared using CFA-Na2SiO3and com-Na2SiO3as additives are shown in Table I. The slurries had similar densities, with slight differences as the amount of additive added increased. Some of the slurries containing CFA-Na2SiO3 had slightly lower densities compared to the slurries containing com-Na2SiO3. There was a 0.02% difference in the densities of the slurries containing 2% additive (CFA-Na2SiO33 and com-Na2SiO3). The difference may be due to the fact that slurries prepared using CFA-Na2SiO3contained a lot of froth. The rheologies of slurries prepared using CFA-Na2SiO3 and com-Na2SiO3 as additives are shown in Tables III and IV, respectively. The slurries prepared using both additives exhibited good rheology, with plastic viscosities (Pv) between 3.75 and 18.75 cp and yield points (Yp) between 13.5 and 61.75 lb/100 ft2. The slurries containing com-Na2SiO3 generally had higher rheological values than those prepared using the CFA-Na2SiO3. At 60 r/min the viscosity of the slurry containing 1% com-Na2SiO3 was 45 cp, while that for the CFA-Na2SiO3 slurry was 26 cp. The slurries prepared using CFA-Na2SiO3 were less viscous and this can be attributed to the presence of froth.
It is known that the use of sodium silicate as a water extender in OWC operation helps to prevent breakdown of weak formations and loss of circulation (Nelson et al., 1990). In addition, it helps to lower the hydrostatic pressure, thereby enhances the 'pumpability' of the cement slurry (Nelson et al., 1990). When sodium silicate is used as a water extender in OWC operation, it reacts with calcium hydroxide in the cement slurry to produce a viscous C-S-H gel that allows addition of large volume of water to the slurry, thereby reducing the density of the slurry and increasing its yield. From the results from the rheology analysis of the CFA-Na2SiO3 prepared from CFA in this study and tested in the formulation of OWC slurries, it is obvious that slurries prepared with CFA-Na2SiO3might be preferable to those prepared with com-Na2SiO3for an OWC operation that requires early strength development.
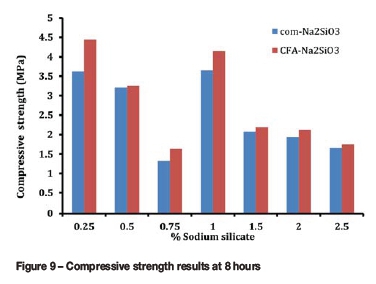
Tables V and VI show the compressive strength results for slurries containing com-Na2SiO3and CFA-Na2SiO3, respectively. As expected, there was a decrease in compressive strength for all slurries as the amount of water added increased. This is consistent with findings from the literature. An increase in water-to-cement ratio results in a dramatic decrease in the compressive strength of the slurries (Fawzi, 2012), Figures 9-11 show that the slurries containing CFA-Na2SiO3had higher compressive strength than those containing com-Na2SiO33 at 8 hours, 12 hours and 24 hours. From Tables V and VI, it can be observed that the CFA-Na2SiO3slurries gained a compressive strength of 0.34 MPa earlier than the Na2SiO3 slurries. This is the minimum strength required to hold the casing in position. In addition, the earlier gain of the compressive strength by the with CFA-Na2SiO3slurries indicates that the slurries with CFA-Na2SiO3set quicker than those with com-Na2SiO3. The results obtained from the ultrasonic cement analyser (UCA) also indicate that the same observation was obtained for slurries that attained a compressive strength of 3.4 MPa within the first 24 hours. A strength of 3.4 MPa is sufficient to hold the casing when further drilling or perforation of the casing is required.


Conclusions
The results indicate that the South African CFA used in this study is a Class F CFA and contains 58% amorphous SiO2. The physico-chemical properties of the synthesized Na2SiO3are consistent with those of the commercial Na2SiO3, indicating the purity of the as-prepared Na2SiO3from the CFA. In addition, the synthesis protocol, which was at a mild temperature, confirms the energy efficiency of the method used. Cement slurries prepared with CFA-Na2SiO3show better performance than those prepared with com-Na2SiO3. Rheological testing indicated that the slurries prepared with CFA-Na2SiO3are less viscous than those prepared with com-Na2SiO3. The CFA-Na2SiO3slurries will thus be easier to pump and handle during OWC operation compared to the slurries prepared with com-Na2SiO3. UCA analysis showed that the slurries prepared with CFA-Na2SiO3have higher compressive strength in comparison to those prepared from the com-Na2SiO3. Although a small concentration of sodium silicate (0.2% to 3.0% BWOC) is always used in OWC operations to yield a reasonable compressive strength (Nelson et al., 1990), the higher compressive strength of the slurries with CFA-Na2SiO3implies that smaller amounts of CFA-Na2SiO3will be required for OWC operations. In addition, slurries prepared with CFA-Na2SiO3will be preferable to those prepared with com-Na2SiO3for an OWC operation that requires early strength development. The results of this study could provide a platform for further research development on the beneficiation of South African CFA in the petroleum, oil, and gas industry.
Acknowledgements
The authors hereby acknowledge the financial support from Chemical Industries Education and Training Authorities (CHIETA) South Africa and Baker Hughes South Africa (Mossel Bay) for availing their cement testing laboratory facility for use in this study.
References
Ahmaruzzaman, M. 2010. A review on the utilization of fly ash. Progress in Energy and Combustion Science, vol. 36, no. 1. pp. 327-363. [ Links ]
Aigbodion, V.S., Hassan, S.B., Ause, T., and Nyior, G.B. 2010. Potential utilization of solid waste (bagasse ash). Journal of Minerals and Materials Characterization and Engineering, vol. 9, no. 1. pp. 67-77. [ Links ]
American Petroleum Institute Specification 10A. 2002. Specifications for Cementing and Materials for Well Cementing. ANSI/API 10A/ISO104261-2001. [ Links ]
ASTMC618. 2012. Standard specification for coal fly ash and raw or calcined natural Pozzolan for use in concrete. [ Links ]
Ayanda, O.S., Fatoki, O.S., Adekola, F.A., and Ximba B. J. 2012. Characterization of fly ash generated from Matla Power Station in Mpumalanga, South Africa. E-Journal of Chemistry, vol. 9, no. 4. pp. 1788-1795. [ Links ]
Essien, E.R., Olaniyi O.A., Adams L.A., and Shaibu R.O. 2011. Highly porous silica network prepared from sodium metasilicate. Journal of Metals, Materials and Minerals, vol. 21, no. 2. pp. 7-12. [ Links ]
Fawzi, R.H. 2012. Thickening time and compressive strength correlations for Bentonitic -class 'G' cement slurries. Iraqi Journal of Chemical and Petroleum Engineering, vol. 13, no. 2. pp. 37-45. [ Links ]
Fisher, G.L., Prentice, B.A., Silberman, D., Ondov, J.M., Biermann, A.H., Ragaini, R C., and McFarland A.R. 1978. Physical and morphological studies of size- classified coal fly ash. Journal of the American Chemical Society, vol. 12, no. 4. pp. 477-451. [ Links ]
Foletto, E.L., Gratieri, E., Hadlichde Oliveira, L., and Jahn S.L. 2006. Conversion of rice hull ash into soluble sodium silicate. Materials Research, vol. 9, no. 3. pp. 335-338. [ Links ]
Ikotun, B.D., Mishra, S., and Fanourakis G.C. 2014. Structural characterization of four South African fly ashes and their structural changes with β-cyclodextrin. Particulate Science and Technology, vol. 32, no. 4. pp. 360-365. [ Links ]
Iyer, R S. and Scott, J.A. 2001. Power station fly ash - a review of value-added utilization outside of the construction industry. Resources, Conservation and Recycling, vol. 31. pp. 217-228. [ Links ]
Joel, O.F. and Ujile, A.A. 2009. Performance evaluation of low density bentonite and econolite cement, Nigeria. International Journal of Natural and Applied Science, vol. 5, no. 4. pp. 322-328. [ Links ]
Kamarudin, R.A., Matlob, A.S., Jubri, Z., and Ramli Z. 2009. Extraction of silica and alumina from coal fly ash for the synthesis of zeolites. Proceedings of ICEE 2009, 3rd International Conference on Energy and Environment, Malacca, Malaysia, 7-8 December 2009. IEEE, New York. [ Links ]
Lea, F.M. 1971. The Chemistry of Cement and Concrete. Chemical Publishing Company Inc., New York, USA. [ Links ]
Mainganye, D., Ojumu, T.V., and Petrik, L. 2013. Synthesis of zeolites Na-P1 from South African coal fly ash: Effect of impeller design and agitation. Materials, vol. 6. pp. 2074-2089. [ Links ]
McDaniel, G.R. 1961. Wet Production of Silicates. US patent no. US2983423. [ Links ]
Miller, F.A. and Wilkins, C.H. 1952. Infrared spectra and characteristic frequencies of inorganic ions: their use in qualitative analysis. Analytical Chemistry, vol. 24, no. 8. pp. 1253-1294. [ Links ]
Music, S., Vincekovic, N.F., and Sekovanic, L. 2011. Precipitation of amorphous SiO2 particles and their properties. Brazilian Journal of Chemical Engineering, vol. 28, no. 1. pp. 89-94. [ Links ]
Nelson, E.B. and Guillot, D. 1990. Well Cementing. 2nd edn. Schlumberger, Texas. [ Links ]
Nelson, E.B., Baret, J.F., and Michaux, M.1990. Well Cementing: Cement Additives and Mechanisms of Action. Elsevier, USA. [ Links ]
Okoronkwo, E.A., Imoisili, P.E., and Olusunle, S.O.O. 2013. Extraction and characterization of amorphous silica from corn cob ash by sol-gel method. Chemistry and Materials Research, vol. 3, no. 4. pp. 68-72. [ Links ]
Park, J., Han, Y., and Kim H. 2012. Formation of mesoporous materials from silica dissolved in various NaOH concentrations: effect of pH and ionic strength. Journal of Nanomaterials, January 2012. Article no. 528174. 10 pp. [ Links ]
Saikia, B.J., Parthasarathy, G., Sarmah, N.C., and Baruah, G.D. 2008. Fourier transform infrared spectroscopic characterization of naturally occurring glassy fulgurites. Bulletin of Material Science, vol. 31, no. 2. pp. 155-158. [ Links ]
Salim, P. and Amani, M. 2013. Special considerations in cementing high pressure high temperature wells. International Journal of Engineering and Applied Sciences, vol.1, no. 4. pp. 1-24. [ Links ]
Shahriar, A. 2011. Investigation on Rheology of Oil Well Cement Slurries. PhD thesis, University of Western Ontario, London, Ontario, Canada. [ Links ]
Tang, C., Thawornsak, N., Samrankrang, S., Jullaphan, O., and Chareonpanich M. 2012. Creating high-value eco-friendly materials from industrial coal combustion ash. Proceedings of the EURO COALASH 2012 Conference, Thessaloniki, Greece. http://www.evipar.org/innet/files/EUROCOALASH2012/Docs/slides/020_Tang_EUROCOALASH2012-paper.pdf [ Links ]
Taylor, H.F.W. 1990. Cement Chemistry. Academic Press, London. [ Links ]
Ying-Mei, X., Ji, Q., De-Min, H., Dong-Mei, W., Hui-Ying, C., Jun, G., and Qiu-Min, Z. 2010. Preparation of amorphous silica from oil shale residue and surface modification by silane coupling agent. Oil Shale, vol. 27, no. 1. pp. 37-48. [ Links ]
Paper received June 2015
Revised paper received Nov. 2015














