Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.12 Johannesburg Dec. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n12a1
PAPERS - CHEMICAL ENGINEERING & METALLURGY AT WITS
Removal of heavy metals using cassava peel waste biomass in a multi-stage countercurrent batch operation
G.S. Simate; S. Ndlovu; L. Seepe
School of Chemical and Metallurgical Engineering, University of the Witwatersrand, Johannesburg
SYNOPSIS
This paper presents a study of the removal of cobalt (Co2+), chromium (Cr3+), and vanadium (V3+) from synthetic effluent solution using cassava waste biomass. Test work was carried out in a multi-stage countercurrent batch system. Single and ternary metal ion systems were studied. A feed inlet concentration of 100 mg/L for each metal ion system was contacted with 0.5 g of cassava waste biomass. The target concentration in the final outlet stream was set against the South African Department of Water and Forestry (DWAF) standards. The results showed that the adsorption capacity was slightly lower for ternary metal ion systems than for single metal ion systems. This was attributed to the greater competition among the metal ions for the occupancy of the binding surfaces on the cassava waste in the ternary systems. Eight adsorption stages were required to meet the targeted limit of 0.5 mg/L for Co2+set by the DWAF. The Cr3+system needed six stages to obtain the targeted limit of 0.1 mg/L, while the V3+ system required four stages to attain the target limit of 0.2 mg/L. In general, cassava waste biomass adsorbed the metal ions in the following order: V3+> Cr3+> Co2+.
Keywords: heavy metals, wastewater, biosorption, cassava waste, biomass, multistage, countercurrent, batch.
Introduction
Wastewaters from many sources, such as the metallurgical, tannery, chemical manufacturing, mining, and battery manufacturing industries, contain toxic heavy metals. The concentrations of some of the toxic metals in these effluents are sometimes higher than permissible discharge levels. Discharge of the contaminated wastewater into the environment would, therefore, create a significant environmental hazard, including impacts on human, animal, and plant health (Matouq et al., 2005). According to the South African Department of Water Affairs and Forestry (DWAF) (2005), the permissible discharge limits for chromium, cobalt, and vanadium are 0.1, 0.5, and 0.2 mg/L, respectively. Therefore, it becomes necessary to remove these heavy metals from wastewaters by an appropriate treatment process before releasing them into the environment (Meena et al., 2005).
Several conventional chemical and physical methods have been developed and used to remove high concentrations of heavy metals from wastewater effluents, including (but not limited to) precipitation, solvent extraction, ion exchange, reverse osmosis, oxidation/reduction, sedimentation, filtration, and electrochemical techniques (Volesky, 2001; Feng et al., 2010; Nassar, 2010; Shen et al., 2009; Song et al., 2011; Ahmadi et al., 2014). However, most of these methods have high capital and operational costs, low metal removal efficiency at low concentrations, and generate toxic sludge that requires additional treatment (Ahmadi et al., 2014; Motouq et al., 2015). Therefore, a lot of effort has been directed at the development of economical methods for the removal of toxic heavy metals from wastewater effluents.
The use of biological-based technologies such as biosorption for removal of heavy metals from wastewater effluents has recently become the subject of considerable interest because of the low cost and high efficiency associated with the process (Arminia et al., 2015). Previous studies by the authors have shown that cassava peel waste is a potentially useful biosorbent for treating wastewater contaminated with Co2+, V3+, and Cr3+ions (Ndlovu et al., 2013; Simate and Ndlovu, 2014; Seepe, 2014). Cassava is a perennial woody shrub, grown as an annual crop, and serves as a major source of low-cost carbohydrates for populations in the humid tropics (O'Hair, 1995; Simate et al., 2013; Adetunji et al., 2015). In the past, the largest producer of cassava was Brazil, followed by Thailand, Nigeria, the DRC, and Indonesia (O'Hair, 1995; Adetunji et al., 2015), but today Nigeria is the largest producer (Adetunji et al., 2015). So far, not much effort has been made to control or manage the enormous quantities of wastes arising from processing cassava tuber into its various products, which are abundant and available in all seasons (Seepe, 2014). Since cassava waste has no economic value, its conversion into biosorbents would ultimately economically benefit the millions of cassava producers. Indeed, the economic utilization of cassava waste would not only provide a solution to the environmental nuisance that it poses, but would create employment and improve local economies (Ndlovu etal., 2013).
Since the manner in which the biomass contacts with the solution to be treated is of particular significance for large-scale treatment of water, initial studies by the authors involved, firstly, batch equilibrium relating to adsorbate, adsorbent, and operating conditions (Ndlovu et al., 2013). This was followed by heavy metal removal studies in a column packed with immobilized cassava waste pellets (Simate and Ndlovu, 2014). Column-type continuous flow operations have an advantage over batch-type operations because the rates of adsorption depend on the concentration of solute in the solution being treated. For column operation, the biomass is continuously in contact with a fresh solution. Consequently, the concentration in the solution in contact with a given layer of biomass in a column changes very slowly. In batch treatment, the concentration of solute in contact with a specific quantity of biomass decreases much more rapidly as adsorption proceeds, thereby decreasing the effectiveness of the adsorbent for removing the solute (Zhou, 2013).
However, the main limitations of the two studies are: (1) batch systems are usually limited to the treatment of small quantities of wastewater (Bharathi and Ramesh, 2013), and data obtained from such systems may not be applicable directly to most treatment systems (such as column operations) where the contact time is not sufficient for the attainment of equilibrium (Zulfadhly et al., 2001; Vinodhini and Das, 2010; Vimala et al., 2011; Bharathi and Ramesh, 2013); and (2) fixed-bed column studies are characterized by clogging and subsequent release of adsorbent into the treated wastewater (Amirnia et al., 2015), and immobilization of biomass also causes mass transfer limitations by hindering the access of the metals to the biosorbent sites compared to suspended biosorbents (Tsezos, 1990; Cassidy et al., 1996). Furthermore, the regeneration capacity of immobilized biomass is limited, thus there is need for biomass to be replaced frequently, which is a costly process (Amirnia et al., 2015). However, continuous operation is the only viable way of treating large volumes of wastewater in a reasonable time, and this is where bench-scale batch biosorption studies are limited in their scope (Amirnia et al., 2015).
Based on the authors' earlier work on the removal of Co2+, V3+, and Cr3+ions using cassava waste biomass in a batch system (Ndlovu et al., 2013), metal adsorption using a multi-stage countercurrent batch system was investigated in this study. Multi-stage countercurrent adsorption operations are superior to both batch and fixed bed operations because countercurrent flow maximizes the average driving force for mass transfer between the fluid and the adsorbent (Seepe, 2014). This technique involved contacting the metal ion solution with the cassava waste biomass in a series of gently agitated tanks for a sufficient retention time. The cassava and metal ion solution were transferred in a countecurrent flow arrangement. The schematic diagram for multi-stage batch adsorption is shown in Figure 1. In multi-stage counter-current batch adsorption, the solution to be treated contains L dm3solution and the concentration of heavy metals is reduced in each stage from Cn-1 to Cn mg/dm3. The amount of biomass added is B g and the heavy metal concentration on the biomass is increased from qn+1to qnmg/g of biomass.
Materials and methods
Preparation ofcassava waste biomass
The cassava tubers were obtained from local wholesalers in South Africa and were prepared as described by Ndlovu et al. (2013). In summary, cassava tubers were carefully peeled and the dried cassava peel waste was ground to 100 urn using a blender. Subsequently, the ground cassava peel waste was treated with nitric acid. Finally, sulphhydryl groups (or thiol groups) were introduced onto the cassava biomass using thioglycolic acid solution in the presence of hydroxy-lamine.
Multi-stage countercurrent biosorption operation
As illustrated in Figure 1, the multi-stage countercurrent biosorption was operated batchwise. About 0.5 g of cassava waste biomass was contacted with 100 mL of the influent solution in each reactor. The concentration of each metal ion in the inlet solution stream was 100 mg/L. Tests were conducted for single and ternary metal ion systems. Mixing was provided by agitation at 150 r/min. After 30 minutes, agitation was stopped and the biosorbent separated from metal ion solution by filtration. The biosorbent and effluent solutions were then transferred to the next respective reactors in a countercurrent manner. This procedure was followed until the final effluent was transferred to the discharge tank from reactor n. The saturated spent biomass emerging from the first reactor was transferred to the regenerator, in which the adsorbed metals were removed, and the biomass was subsequently reactivated and then returned to the adsorption circuit. The main advantage of this process is that the adsorbent can be regenerated as soon as its role in the adsorption step has been completed. Thus, in theory at least, the inventory of the adsorbent can be kept to a minimum. The quantity of adsorbent required for a given separation can also be reduced by increasing the number of stages. However, the possibility of regeneration and re-use of the cassava biomass was beyond the scope of this study. In practice, the heavy metals eluted from the cassava biomass could be further concentrated and/or used as an input in other hydrometallurgical processes.
Results and discussion
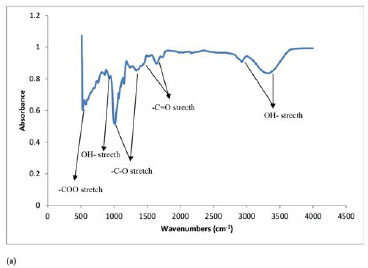
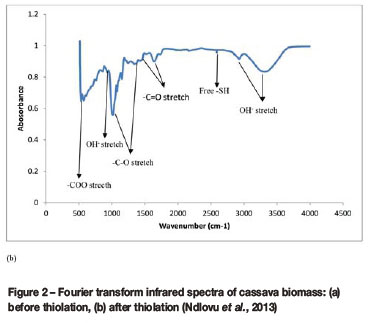
Fourier transform infrared (FTIR) spectroscopy was used to identify the presence or absence of functional groups on the surface of cassava peel waste biomass before and after thiolation. Figure 2 shows the obtained FTIR spectra. As can be seen from Figure 2(a) and (b), the major difference between the two biomasses is the presence of the sulphhydryl group (-SH), which proves that thiolation process adds sulphhydryl functional groups on the cassava peel biomass (Ndlovu et al., 2013; Simate and Ndlovu, 2015). Other differences between treated and untreated cassava biomass were reported by Ndlovu et al. (2013). For example, the BET surface areas obtained were in the following order: untreated < 0.5 M acid treated < 1 M treated, and both the pore volume and pore size were in the following order: untreated > 0.5 M acid treated > 1 M treated. The point of zero charge (pzc) for the untreated and treated cassava peel biomass was determined as 4.9 and 3.1, respectively. This shows that the functional groups on untreated cassavapeel biomass are weakly acidic (or more basic) than the functional groups on treated cassava peel biomass. Therefore, the pzc results confirm that more acidic functional groups were incorporated into the cassava peel biomass during thiolation. These results concurr well with the results shown in Figure 2(b), which shows the introduction of an acidic sulphhydryl group after thiolation.
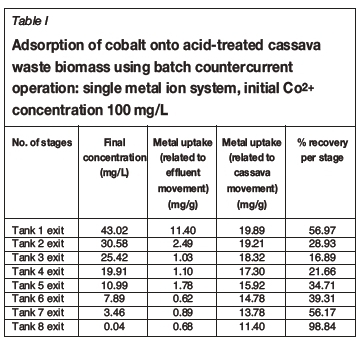
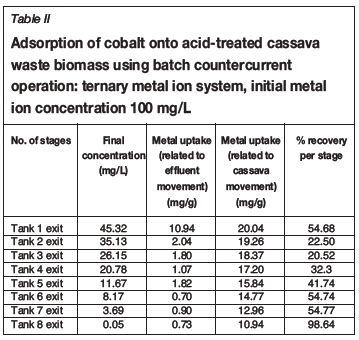
Tables I-VI show the results of a series of adsorption tests, and the number of stages required to reduce the heavy metals in this study to acceptable drinking water standards as set by the DWAF. Tables I and II show the adsorption of Co2+in single and ternary systems, respectively. Eight stages of Co2+removal were needed to meet the targeted limit of 0.5 mg/L in the effluent discharge. The effluent solution obtained after the 8th stage contained 0.04 and 0.05 mg/L Co2+for single and ternary systems, respectively. Tables III and IV show that the adsorption of Cr3+ needed six stages to obtain the targeted limit of 0.1 mg/L. For single system and ternary system, 0.04 and 0.05 mg/L were obtained, respectively. For V3+, the effluent solution at the end of the 4th stage contained 0.01 and 0.02 mg/L for single and ternary systems, respectively (see Tables V and VI), with a target limit of 0.2 mg/L. In all cases, the adsorption capacity was slightly lower for the ternary metal ion system as compared to the single metal ion system. This may be attributed to the greater competition between the metal ions for the occupancy of the binding surfaces on the cassava waste biomass (Ndlovu et al., 2013). Generally, the biosorption efficiency increased in the order Co2+ < Cr3+ < V3+. These results are in agreement with our previous batch studies (Ndlovu et al., 2013) and column studies (Simate and Ndlovu, 2014). The differences (or variations) are attributed to the metal ions' affinity towards the biosorbent (Mohan and Sreelakshmi, 2008), and this depends on the ionic radius and electropositive charges on the ions (Reddad et al., 2002).
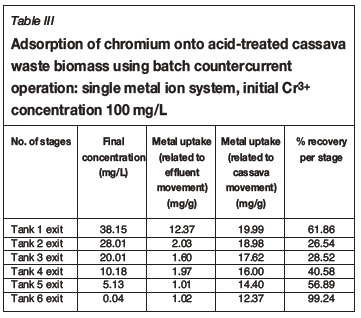
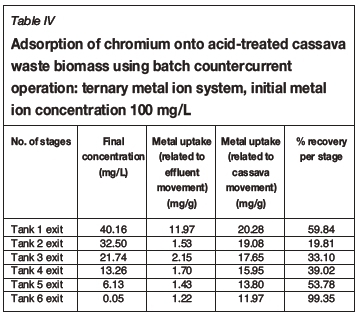
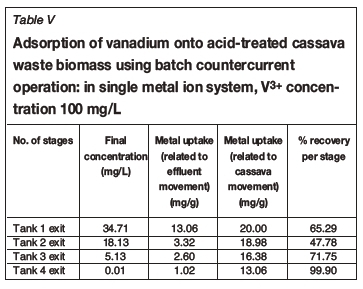
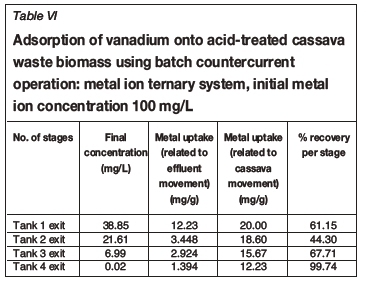
This study showed that the adsorption efficiency obtained in the multi-stage countercurrent system is higher than that obtained in our study of the batch system, implying that there is a limited number of active binding sites on the adsorbent for metal adsorption in a batch system. However, in the multi-stage system, a somewhat weak solution is in contact with a biomass that has more active binding sites as it moves up the train from last stage to the first stage, hence higher recoveries were obtained.
Conclusions
The required discharge limits were obtained for all metals using multi-stage countercurrent batch adsorption system with cassava peel waste biomass as biosorbent. The minimal accepted limits for Co2+, Cr3+and V3+discharge were reached in 8, 6, and 4 stages, respectively. Thus, in general, cassava waste biomass adsorbed the metal ions in the following order: V3+> Cr3+ > Co2+. The adsorption efficiency obtained in the multi-stage countercurrent system was higher than that achieved previously in a batch process.
Acknowledgements
The authors are thankful to all those who provided financial [NRF Scholarship (South Africa) and Friedel Sellschop award (University of the Witwatersrand, South Africa)] or technical support in the course of this research work. The work is part of the MSc (Eng) dissertation submitted by Ms Lizzy Seepe in 2014 to the University of the Witwatersrand, Johannesburg.
Disclaimer
The contents of this paper reflect the views of the authors, who are responsible for the facts and accuracy of the data presented herein and do not necessarily reflect the official views or policies of any agency or institute. This paper does not constitute a standard or specification, nor is it intended for design, construction, bidding, or permit purposes. Trade names were used solely for information and not for product endorsement.
References
Adetunji, A.R., Isadare, D.A., Akinluwade, K.J., and Adewoye, O.O. 2015. Waste-to-wealth applications of cassava - A review study of industrial and agricultural applications. Advances in Research, vol. 4, no. 4. pp. 212-229. [ Links ]
Ahmadi, A., Heidarzadeh, S., Mokhtari, A.R., Darezereshki, E., and Harouni, H.A. 2014. Optimization of heavy metal removal from aqueous solutions by maghemite (y-Fe2O3) nanoparticles using response surface methodology. Journal of Geochemical Exploration, vol. 147. pp. 151-158. [ Links ]
Amirnia, S., Ray, M.B., and Margaritis, A. 2015. Heavy metals removal from aqueous solutions using Saccharomyces cerevisiae in a novel continuous bioreactor-biosorption system. Chemical Engineering Journal, vol. 264, pp. 863-872. [ Links ]
Bharathi, K.S. and Ramesh, S.P.T. 2013. Fixed-bed column studies on biosorption of crystal violet from aqueous solution by Citrullus lanatus rind and Cyperus rotundus. Applied Water Science, vol. 3, no. 4. pp. 673-687. [ Links ]
Cassidy, M.B., Lee, H., and Trevors, J.T. 1996. Environmental applications of immobilized microbial cells: A review. Journal of Industrial Microbiology, vol. 16. pp. 79-101. [ Links ]
Department of Water Affairs and Forestry (DWAF). 2005. A drinking water quality framework for South Africa. http://www.orangesenqurak.org/UserFiles/File/OtherV2/Drinking%20Water%20Quality%20Framework%20for%20South%20Africa%20DWAF%202005.pdf [Accessed May 2014]. [ Links ]
Feng, Y., Gong, J.-L., Zeng, G.-M., Niu, Q.-Y., Zhang, H.-Y., Niu, C.-G., Deng, J.-H., and Yan, M. 2010. Adsorption of Cd (II) and Zn (II) from aqueous solutions using magnetic hydroxyapatite nanoparticles as adsorbents. Chemical Engineering Journal, vol. 162. pp. 487-494. [ Links ]
Matouq, M., Jildeh, N., Qtaishat, M., Hindiyeh, M., and Syouf, M.Q. 2015. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. Journal of Environmental Chemical Engineering, vol. 3. pp. 775-784. [ Links ]
Meena, A.K., Mishra, G.K., Rai, P.K., Rajagopal, C., and Nagar, P.N. 2005. Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent. Journal of Hazardous Materials, vol. 122. pp. 161-170. [ Links ]
Mohan, S. and Sreelakshmi, G. 2008. Fixed bed column study for heavy metal removal using phosphate treated rice husk. Journal of Hazardous Materials, vol. 153. pp. 75-82. [ Links ]
Nassar, N.N. 2010. Rapid removal and recovery of Pb (II) from wastewater by magnetic nanoadsorbents. Journal of Hazardous Materials, vol. 184. pp. 538-546. [ Links ]
Ndlovu, S., Simate, G.S., Seepe, L., Shemi, A., Sibanda, V., and van Dyk, L. 2013. Removal of Co2+, V3+ and Cr3+ from synthetic effluents using cassava waste. South African Journal ofChemical Engineering, vol. 18, no. 1. pp. 1-19. [ Links ]
O'Hair, S.K., 1995. Cassava. https://hort.purdue.edu/newcrop/CropFactSheets/cassava.html [Accessed November 2015]. [ Links ]
Reddad, Z., Gerente, C., Andres, Y., and Le Cloirec, P. 2002. Adsorption of several metal ions onto a low-cost biosorbent: Kinetic and equilibrium studies. Environmental Science and Technology, vol. 36. pp. 2067-2073. [ Links ]
Seepe, L. 2014. The use of cassava waste in the removal of cobalt, chromium and vanadium metal ions from synthetic effleunts. MSc (Eng) dissertation, university of the Witwatersrand, Johannesburg. [ Links ]
Shen, Y., Tang, J., Nie, Z., Wang, Y., Ren, Y., and Zuo, L. 2009. Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Separation and Purification Technology, vol. 68. pp. 312-319. [ Links ]
Simate, G.S., Ndlovu, S., Iyuke, S.E., and Walubita, L.F. 2015. Biotechnology and nanotechnology: A means for sustainable development in Africa. Chemistry for Sustainable Development in Africa.. Gurib-Fakim, A. and Eloff, J.N. (eds). Springer-Verlag, Berlin. pp. 159-191. [ Links ]
Simate, G.S. and Ndlovu, S. 2015. The removal of heavy metals in a packed bed column using immobilized cassava peel waste biomass. Journal of Industrial and Engineering Chemistry, vol. 21. pp. 635-643. [ Links ]
Song, J., Kong, H., and Jang, J. 2011. Adsorption of heavy metal ions from aqueous solution by polyrhodanine-encapsulated magnetic nanoparticles. Journal of Colloid and Interface Science, vol. 359. pp. 505-511. [ Links ]
Tsezos, M. 1990. Engineering aspects of metal binding by biomass. Microbial Mineral Recovery. Ehrlich, H.L. and Brierly, C.L. (sds). McGraw-Hill, New York. [ Links ]
Vimala, R., Charumathi, D., and Das, N. 2011. Packed bed column studies on Cd(II) removal from industrial wastewater by macrofungus Pleurotus platypus. Desalination, vol. 275. pp. 291-296. [ Links ]
Vinodhini, V. and Das, N. 2010. Packed bed column studies on Cr (VI) removal from tannery wastewater by neem sawdust. Desalination, vol. 264. pp. 9-14. [ Links ]
Volesky, B. 2001. Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy, vol. 59. pp. 203-216. [ Links ]
Zhou, Y. 2013. XXIVth International Congress of Pure and Applied Chemistry. oxford, Butterworth-Heinemann. [ Links ]
Zulfadhly, Z., Mashitah, M.D., and Bhatia, S. 2001. Heavy metal removal in fixed bed column by the macrofungus Pycnoporus sanguineus. Environmental Pollution, vol. 112. pp. 463-470. [ Links ]
Paper received May 2015
Revised paper received Nov. 2015.