Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.10 Johannesburg Oct. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n10a10
A theoretical approach to the sublimation separation of zirconium and hafnium in the tetrafluoride form
H.F. NiemandI; P.L. CrouseII
IThe South African Nuclear Energy Corporation SOC Ltd. (Necsa)
IIDepartment of Chemical Engineering, University of Pretoria
SYNOPSIS
The separation of zirconium and hafnium is essential in the nuclear industry, since zirconium alloys for this application require hafnium concentrations of less than 100 ppm. The separation is, however, very difficult due to the numerous similarities in the chemical and physical properties of these two elements.
Traditional methods for separation of zirconium and hafnium rely predominantly on wet chemical techniques, absolvent extraction. In contrast to the traditional aqueous chloride systems, the AMI zirconium metal process developed by Necsa focuses on dry fluoride-based processes. Dry processes have the advantage of producing much less hazardous chemical waste.
In the proposed AMI process, separation is effected by selective sublimation of the two tetrafluorides in an inert atmosphere under controlled conditions, and subsequent selective desublimation. Estimates are made for the sublimation rates of the two tetrafluorides based on the equilibrium vapour pressures. A sublimation model, based on the sublimation rates, was developed to determine if the concept of separation by sublimation and subsequent desublimation is theoretically possible.
Keywords: sublimation separation, zirconium tetrafluoride, hafnium tetrafluoride.
Introduction
Zirconium requires several purification steps to conform to nuclear-grade specifications. Little information is available on the sublimation separation of Zr and Hf compounds, especially in the fluoride form, the majority of which deals with sublimation under vacuum conditions. On the industrial scale, only vacuum sublimation of ZrF4 has been reported. No records were found for the sublimation of ZrF4 in an inert atmosphere. Information on the sublimation rate of ZrF4 or HfF4 in an inert atmosphere is also limited. The rate is assumed to be dependent on several factors, of which temperature, area, and composition are considered the most important.
In the process currently under investigation, the separation technique envisaged is by sublimation/desublimation in the tetrafluoride form. The separation involves the sublimation of the metal tetrafluorides in an inert atmosphere under controlled conditions, followed by desublimation (i.e. condensation) of the one metal fluoride in preference to the other.
In this paper, a sublimation model is developed to predict the sublimation rates of both ZrF4 and HfF4 in an inert gas. These rates are used to calculate the partial pressures of the two fluorides exiting a sublimer and entering a desublimer where the one tetrafluoride is selectively removed from the gas stream, thereby separating the two tetrafluorides.
Literature survey and theoretical discussion
Sublimation methods used for the separation of Zr and Hf are reported in literature, but these methods are all under vacuum conditions (Monnahela et al., 2013; Solov'ev and Malyutina, 2002a).
Sublimation is, however, a general method used for the purification of ZrF4 by removing most trace elements, e.g. Fe, Co, Ni, and Cu (Abate and Wilhelm, 1951; Dai et al., 1992; Kotsar' et al., 2001; MacFarlane et al., 2002; Pastor and Robinson, 1986; Solov'ev and Malyutina, 2002b; Yeatts and Rainey, 1965).
The addition of baffles (Abate and Wilhelm, 1951; Kotsar' et al., 2001; Yeatts and Rainey, 1965) is used quite frequently to help reduce the mechanical carry-over of impurities. These baffles are merely plates positioned between the charge and the cold finger. These impurities impart a greyish colour to ZrF4, whereas pure ZrF4 is much whiter.
The literature also describes the use of a gettering agent (Monnahela et al., 2013; Solov'ev and Malyutina, 2002a), which seems to reduce the number of steps required to produce nuclear-grade ZrF4. Getters used include NiF2, zirconium oxides, and/or zirconium oxyfluorides.
Area-dependent sublimation rate
MacFarlane et al. (2002) calculated the area-dependent rate of sublimation of ZrF4 and obtained a value of approximately 1.87 g/m2/s at 850-875°C.
Product composition
Ti, Esyutin, and Scherbinin (1990a, 1990b) found that pure ZrF4 has a higher sublimation rate than industrial-grade ZrF4, which contains a degree of impurities. They concluded that this might be due to the accumulation of low-volatile components in the near-surface layer of the sample, making diffusion and evaporation increasingly difficult and resulting in decreased sublimation flux.
Layer height
In a study on the influence of layer height on the vacuum sublimation rate of ZrF4, Ti, Esyutin, and Scherbinin (1990c) concluded that the sublimation rate does not necessarily depend on the height of the sample in the sublimator.
Vapour pressure of ZrF4
Figure 1 gives a range of vapour pressures from the literature for both ZrF4 and HfF4 at temperatures above 600°C (Benedict et al., 1981; Cantor et al., 1958; Koreneo et al., 1972; Sense et al., 1954, 1953).
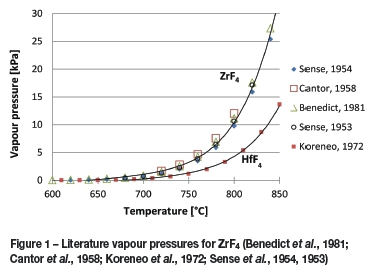
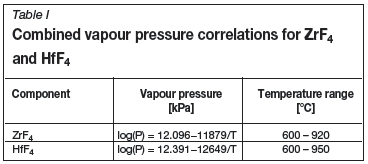
The data in Figure 1 was combined and can be expressed as Antoine constants for both ZrF4 and HfF4. These two expressions are given in Table I.
Experimental concept to be modelled
The flow diagram for the proposed process is presented in Figure 2. The concept under consideration is to pass a stream of pre-heated dry nitrogen as a carrier gas over a bed of subliming ZrF4 and HfF4 (Figure 3); the gas exiting the sublimer then enters a desublimer operating at a slightly lower temperature. The difference between the vapour pressures as function of temperature is used to determine an optimum temperature for the desublimer. In the desublimer, one of the tetrafluorides is desublimed in preference to the other, thus effecting separation. The remainder of the gas mixture exits the desublimer and enters a water-cooled cold finger for collection of the remaining ZrF4 and HfF4, which can be subjected to a further separation step.
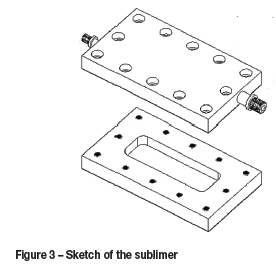
The sublimer (Figure 3) consists of two rectangular sections; a bottom section containing the bulk mass to be sublimed, and a top section that facilitates the movement of the nitrogen gas and carries the sublimed tetrafluorides in the gas phase to the desublimer.
The desublimer is a long cylindrical pipe that is heated to a predetermined temperature, depending on the partial pressures of the ZrF4 and HfF4 entering the desublimer.
Modelling
Flux model
The rate model for the sublimation of ZrF4 and HfF4 is based on the work of Smith (2001), who predicted evaporation rates for liquid spills of chemical mixtures by employing vapour-liquid equilibrium. As sublimation progresses, the bed height decreases with time, which causes changes in the mass transfer coefficient resulting in a change in the sublimation rate of the two tetrafluorides. The rate model for both ZrF4 and HfF4 is given in Equation [1]:
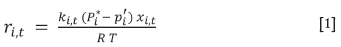
where riis the sublimation rate of ZrF4 (or HfF4) in mol per unit sublimation area per unit time, kitis the mass transfer coefficient in m/s at time t, Piis the vapour pressure in kPa, piis the partial pressure in the bulk gas, Xi>tis the mol fraction of the respective tetrafluoride in the unsublimed bulk mass, R is the ideal gas constant (8.314 kPa.m3/kmol/K), and T is the temperature in K.
In order to calculate the total flux along the length of the sublimation pan the pan is divided into segments each of length M. The flux in each successive segment is calculated by adding the flux in the previous segment to the sublimed masses of ZrF4 and HfF4 in segmentj (Equation [2]).
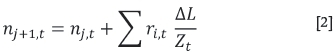
where Ztis the height of the headspace above the solid bed at any given time t.
Model parameters
Mass transfer coefficient
The mass transfer coefficient (ki) is required for calculating the rate of sublimation and is a function of the Sherwood number (Shi), the diffusion coefficient (Dab), and the equivalent flow diameter (De) (Equation [3]):

Sherwood numbers differ for each experimental set-up. In the case of convective mass transfer for forced convection over a flat plate (in this case a sublimation pan), and for laminar flow conditions with Reynolds number < 5 χ 105, Prandtl number > 0.6, and Shmidt number (Sc/) > 0.5, the Sherwood number can be calculated using Equation [4] (Çengel, 2006).
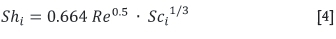
Diffusion coefficients
The diffusion coefficient can be estimated using the Lennard-Jones potential to evaluate the influence of the molecular forces between the molecules. This correlation (Equation [5]), also known as the Chapman-Enskog equation, holds for binary gas mixtures of non-polar, non-reacting species (Green, 2008; Welty, 2001), which is the case for ZrF4 and HfF4 in nitrogen.
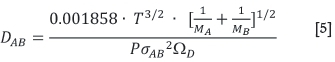
σΑΒis the collision diameter, a Lennard-Jones parameter in Â, where A refers to nitrogen and B to either ZrF4 or HfF4. Since σis denoted as the Lennard-Jones diameter of the respective spherical molecule (Welty, 2001), an estimation was made for the diameter of a ZrF4 and a HfF4 molecule assuming sphericity. The sizes of the respective molecules were calculated at room temperature with the use of SpartanTM software. The equilibrium geometry was calculated using the Hartree-Fock method with the 6 31* basis set. Estimated values for the collision diameters of ZrF4 and HfF4 with N2 were calculated as 4.146 and 4.127 Â respectively.
The collision integral (QD) is a dimensionless parameter and a function of the Boltzmann constant Κ (1.38 χ 10-16 ergs/K), the temperature, and the energy of molecular interaction ЄAB. The boiling points (Tb) for ZrF4 (912°C) and HfF4 (970°C) (Lide, 2007) were used to calculate the values for Eiwith the use of an empirical correlation, given by Equation [6]:

Estimated values for the collision integrals for ZrF4 and HfF4 in N2 were calculated as 0.980 and 0.987, respectively.
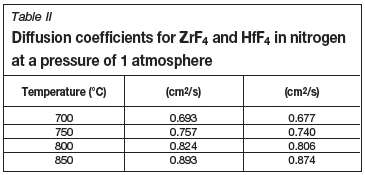
The diffusion coefficients were calculated at several temperatures and are listed in Table II.
Model results
Limitations
One practical problem encountered in the design of the experimental set-up is the working temperature of the valves (650°C). At this temperature, the vapour pressures are relatively low, which results in a very long sublimation time. Since all of the components of the valves are metallic, and it is general knowledge that these valves have a built-in safety factor, the decision was made to operate slightly above the maximum specification temperature of the valves, i.e. at 700°C.
Sublimation rates
The initial load to be sublimed was taken as 80 g, which includes the HfF4 and other impurities. The full sublimation area is 0.0075 m2.
The average area-dependant rates of sublimation at several temperatures calculated from the model are given in Figure 4, which is a sum of the average rates of ZrF4 and HfF4 at several time intervals. It can be seen that the rate is a power function of the temperature, illustrating the effect of a higher temperature on the rate.
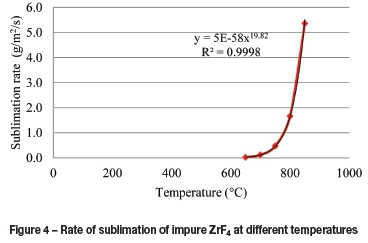
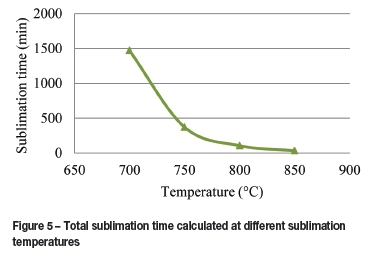
The total sublimation time is dependent on the area of sublimation and the temperature of sublimation, the latter controls the two vapour pressures. The dependence of the total sublimation time on temperature is shown in Figure 5. At 850°C, the total sublimation time equals approximately 33 minutes, whereas at 700°C the total sublimation time was calculated to be approximately 24.5 hours. The vapour pressure ratio between 850 and 700°C is 42.7, which indicates that the rate at 700°C should be lower by at least this factor, which amounts to a total sublimation time of 23.5 hours. The other factor influencing the rate is the partial pressure in the gas stream, which is also higher at the higher temperature, which may contribute to the difference in the total sublimation time at 700°C.
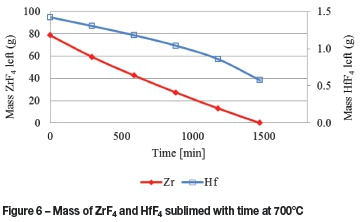
The mass sublimed with respect to time at 700°C is presented in Figure 6. Here it is evident that, according to the model calculations, some HfF4 will remain in the sublimation pan once all the ZrF4 has sublimed. Theoretically this implies that the sublimation can be stopped after a certain time, thereby separating most of the HfF4 from the ZrF4 in the cold finger.
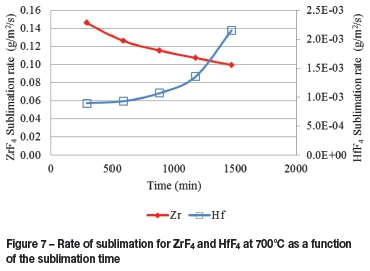
The rates of both HfF4 and ZrF4 sublimation are given in Figure 7. From this figure it is evident that the sublimation rate of HfF4 becomes increasingly significant as the sublimation progresses, i.e. as the bed height lowers with time. This is probably due to the mass fraction HfF4 increasing, since the ZrF4 has a higher rate of sublimation.
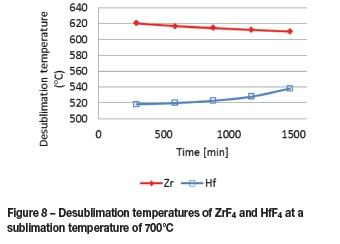
The sublimed ZrF4 and HfF4 diffuse into the nitrogen stream, exit the sublimer, and enter a desublimer operating at a slightly lower temperature than that of sublimation. The desublimation temperatures for the two tetrafluorides can be calculated from the vapour pressure correlations based on the partial pressure of the respective fluorides entering the desublimer. Figure 8 indicates the desublimation temperatures obtained from the model at a sublimation temperature of 700°C for ZrF4 and HfF4. It is evident that a desublimer operating temperature of between 540°C (maximum temperature for HfF4) and 610°C (minimum temperature for ZrF4 desublimation) is required to ensure that, according to the model calculations, no HfF4 will desublime in the desublimer.
ZrF4 lost to cold finger
In the case of ZrF4, the desublimer operating temperature is still relatively high for all the ZrF4 to desublime while passing through the desublimer.
A desublimer operating temperature of 30°C higher than that of the maximum temperature for HfF4(i.e. 570°C) results in a vapour pressure of ZrF4 which is still relatively large, and some of the ZrF4 will therefore not desublime and will be collected on the cold -finger.
Comparison to literature data
The area-dependent rates of sublimation for both ZrF4 and HfF4 at 850°C were calculated. The average rate over the entire duration of sublimation of the total sublimated mass amounts to approximately 5.36 g/m2/s, which is 2.87 times higher than the value estimated from literature data (1.87 g/m2/s). The difference between the literature and model rates may be attributable to the impurities present in the sample used by Macfarlane et al., (2002), since the presence of impurities can have a direct influence on the rate of sublimation.
Conclusions
A sublimation model has been developed to predict the sublimation rates and the partial pressures of ZrF4 and HfF4 in the tetrafluoride form and in an inert gas. The gas exits a sublimer and enters a desublimer where the one tetrafluoride is desublimed in preference to the other, separating the two tetrafluorides.
The model revealed an area-dependent sublimation rate that is 2.87 times higher than the value estimated from literature data (1.87 g/m2/s) at 850°C. This indicates that the rates obtained from the model are within an acceptable range. The difference between the literature and model rates may be attributable to the impurities present in the sample used by Macfarlane et al., (2002), since impurities can have a direct influence on the rate of sublimation.
Due to experimental/equipment constraints, the operating temperature of the sublimer should be in the range of 700°C. Optimal temperature selection is imperative, since low temperatures result in a low sublimation rate and high temperatures increase the level of impurities in the sublimed product.
At a sublimer operating temperature of 700°C, the model indicates that an operating temperature for the desublimer of between 540°C (maximum temperature for HfF4) and 610°C (minimum temperature for ZrF4 desublimation) is required to ensure that, according to the model calculations, only ZrF4 and no HfF4 will desublime. Selection of an optimal temperature of the desublimer is also critical, since too high a temperature will result in more ZrF4 lost to the cold finger.
It is recommended that the model results be compared with experimental values, including if necessary the sublimation kinetics to account for any possible time dependencies of the vapour pressures.
References
Abate, L.J. and Wilhelm, H.A. 1951. Sublimation of zirconium tetrafuoride. US Atomic Energy Commission, Ames Laboratory. [ Links ]
Benedict, M., Pigford, T.H., and Levi, H.W. 1981. Nuclear Chemical Engineering, McGraw-Hill, New York. [ Links ]
Çengel, Y.A. 2006. Heat and Mass Transfer. McGraw-Hill, New York. [ Links ]
Dai, G., Huang, J., Cheng, J., Zhang, C., Dong, G., and Wang, K. 1992. A new preparation route for high-purity ZrF4. Journal of Non-Crystalline Solids, vol. 140, no. 1-3. pp. 229-232. [ Links ]
Cantor, S., Newton, R., Grimes, W., and Blankenship, F. 1958. Vapor pressures and derived thermodynamic information for the system RbF-ZrF4. Journal of Physical Chemistry vol. 62, no. 1. pp. 96-99. [ Links ]
Green, J.D. 2000. II / distillation / sublimation. Encyclopedia of Separation Science. 2nd edn). Wilson, I.D. (ed.). Academic Press, Oxford. [ Links ]
Koreneo, Y., Sorokin, I., Chirina, N., and Novoselo, A.V. 1972. Vapor-pressure of hafnium tetrafluoride. Journal of Inorganic Chemistry, vol. 17, no. 5. pp. 1195. [ Links ]
Kotsar', M.L,. Bateev, V.B., Baskov, P.B., Sakharov, V.V., Fedorov, V.D., and Shatalov, V.V. 2001. Preparation of high-purity ZrF4 and HfF4 for optical fibers and radiation-resistant glasses. Inorganic Materials, vol. 37, no. 10. pp.1085-1091. [ Links ]
Lide, D.R. 2008. CRC Handbook of Chemistry and Physics. CRC, London. [ Links ]
MacFarlane, D.R., Newman, P.J., and Voelkel, A. 2002. Methods of purification of zirconium tetrafluoride for fluorozirconate glass. Journal of the American Ceramic Society, vol. 85, no. 6. pp. 1610-1612. [ Links ]
Monnahela, O.S., Augustyn, W.G., Nel, J.T., Pretorius, C.J., and Wagener, J.B. 2013. The vacuum sublimation separation of zirconium and hafnium tetrafluoride. AMI Precious Metals 2013 Conference, Protea Hotel, Cape Town, 14-16 october 2013. [ Links ]
Nel, J.T., Du Plessis, W., Crous, P.L., and Retief, W.L. 2011. Treatment of zirconia-based material with ammonium bi-fluoride. Patent WO2011013085 A1. [ Links ]
Pastor, R.C. and Robinson, M. 1986. Method for preparing ultra-pure zirconium and hafnium tetrafluorides. US patent 4,578,252 A. [ Links ]
Sense, K.A., Snyder, M.J. and Clegg, J.W. 1953. Vapor pressures of beryllium fluoride and zirconium fluoride. US Atomic Energy Commission Technical Information Services, Oak Ridge, Tennessee. [ Links ]
Sense, K.A., Snyder, M.J., and Filbert, R.B.J. 1954. The vapor pressure of zirconium fluoride. Journal of Physical Chemistry, vol. 58, no. 11. pp. 995-996. [ Links ]
Smith, R.L. 2001. Predicting evaporation rates and times for spills of chemical mixtures. Annals of Occupational Hygiene, vol. 45, no. 6. pp. 437-445. [ Links ]
Solov'ev, A.I. and Malyutina, V.M. 2002a. Production of metallic zirconium tetrafluoride purified from hafnium to reactor purity. Russian Journal of Non-Ferrous Metals, vol. 43, no. 9. pp. 14-18. [ Links ]
Solov'ev, A.I. and Malyutina, V.M. 2002b. Metallurgy of less-common and precious metals. Production of metallurgical semiproduct from zircon concentrate for use in production of plastic metallic zirconium. Russian Journal of Non-Ferrous Metals, vol. 43, no. 9. pp. 9-13. [ Links ]
Ti, V.A., Esyutin, V.S., and Scherbinin, V.P. 1990a. The dependence of the zirconium tetrafluoride sublimation rate in vacuum upon the process temperature and product composition. Kompleksnoe Ispol'zovanieMineral'nogo Syr'ya, vol. 9. pp. 63-64. [ Links ]
Ti, V.A., Esyutin, V.S., and Scherbinin, V.P. 1990b. The influence of the sample height on the zirconium tetrafluoride sublimation process. Kompleksn. Ispol'z. Miner. Syr'ya, vol. pp. 60-61. [ Links ]
Ti, V.A., Esyutin, V.S., and Scherbinin, V.P. 1990c. The dependence of thezirconium tetrafluoride sublimation rate upon the process pressure. Kompleksnoe Ispol'zovanie Mineral'nogo Syr'ya, vol. 10. pp. 61-63. [ Links ]
Welty, J.R., Wicks, C.E., Wilson, R.E. and Rorrer, G. 2001. Fundamentals of Momentum, Heat and Mass Transfer. Wiley, New York. [ Links ]
Yeatts, L.B. and Rainey, W.T. 1965. Purification of zirconium tetrafluoride. Technical report ORNL-TM-1292, US Atomic Energy Commission. [ Links ]
Paper received Mar. 2015
Revised paper received July 2015














