Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.10 Johannesburg Oct. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n10a7
Plasma technology for the manufacturing of nuclear materials at Necsa
I.J. van der Walt; J.T. Nel; J.L. Havenga
The South African Nuclear Energy Corporation SOC Ltd. (Necsa), Pretoria
SYNOPSIS
The development of plasma technology at Necsa started in the early 1980s, when the applicability of high-temperature plasmas in the nuclear fuel cycle was investigated. Since 1995, this plasma expertise has expanded to other industrial applications, for example mineral beneficiation, nanotech-nology, fluorocarbon production and waste treatment, all of which are also of relevance to the nuclear industry.
Necsa has demonstrated the manufacture of plasma-dissociated zircon, zirconium metal powder, carbon nanotubes, silicon carbide (SiC), zirconium carbide (ZrC) and boron carbide (B4C) at the laboratory and pilot plant scale. These materials are commonly used in the nuclear industry. Zirconium alloys are used as fuel cladding material for nuclear fuel assemblies.
Necsa manufactured the monomer tetrafluoroethylene (TFE), using 150 and 450 kW DC plasma systems, from which the polymer polytetraflu-oroethylene (PTFE) was synthesized for use in filters and as seals in nuclear plants. With the nuclear renaissance at hand, it was demonstrated that plasma technology can be used to produce hydrofluoric acid (HF), which is used in the manufacture of fluorine gas (F2) for the production of uranium hexafluoride (UF6) directly from the mineral calcium fluoride (CaF2) without the use of sulphuric acid as in the conventional process. The recovery of valuable uranium from nuclear waste such as filters, oils, and solids with plasma processes will also be discussed. The destruction of low-level nuclear waste by a plasma gasification system can reduce the volume of this waste by several orders in magnitude, resulting in huge savings in the storage costs. Another product of plasma technology is the encapsulation process for nuclear waste and the production of vitrified product, which could be used as filler material for medium-level nuclear waste.
Keywords: plasma, zirconium, fluorocarbons, nuclear waste, nanomaterials.
Introduction
During the 1980s, a plasma research and development programme was launched at the erstwhile Uranium Enrichment Corporation of South Africa (UCOR) at Valindaba outside Pretoria. The main purpose of this programme was to develop alternative and more economical processes for the processing and manufacturing of uranium compounds used in the nuclear fuel cycle. The conversion of UO2 (as received from uranium mines) to UF6, which is needed for enrichment, conventionally involves several laborious, expensive, chemical processing steps. Plasma processes can eliminate several of the intermediate steps.
Enriched UF6 is converted to UO2, which is pressed into pellets that are used in nuclear power plants. Plasma technology could also be used in a modified and optimized conversion process.
Zirconium alloys are used as nuclear fuel cladding material. In the 1980s, UCOR developed plasma process to produce zirconium metal from ZrCl4, using hydrogen as a reductant (Nel et al., 2011).
With the termination of South Africa's nuclear programme in the 1990s, the South African government asked the then Atomic Energy Corporation (AEC) to investigate the possible re-aligning of already established plasma technology, expertise and equipment to investigate other applications for plasma technology in the non-nuclear industry. From this the so-called Metox (Metal Oxide) programme was born. This programme investigated alternative means of zircon beneficiation, as well as the production of high-temperature-resistant ceramic compounds that are used in the nuclear industry, such as alumina, zirconia, silica, silicon carbide, zirconium carbide, and boron carbide. By changing the process parameters, nanoparticles of the abovementioned products were also synthesized in a plasma system.
Polytetrafluoroethylene (PTFE) was extensively used in nuclear plants as seals, in valves and pipes, as containers, filters, etc. PTFE filters used in the enrichment plant that are contaminated with uranium products can be destroyed with a plasma process and simultaneously, in the same process, the uranium values can be recovered.
There is no PTFE or fluoropolymer production facility in South Africa and all these products are imported. In 1994, Necsa started a project on the production of TFE (C2F4), the precursor for PTFE, using a plasma process.
South Africa is the world's fourth-largest producer of calcium fluoride (fluorspar or CaF2), but very little or no local beneficiation takes place. In the nuclear fuel cycle, hydrofluoric acid is produced from CaF2 to manufacture fluorine gas, which is used to make UF6 for the enrichment purposes. It was proved that HF can be manufactured directly in a plasma system instead of using the conventional sulphuric acid route. The destruction, encapsulation, and vitrification of nuclear waste can also be accomplished with a plasma process.
Under the Advanced Metals Initiative (AMI) programme of the Department of Science and Technology (DST), a new process was developed to make nuclear-grade zirconium metal in a continuous plasma process.
The purpose of this paper is to discuss the abovementioned processes in more detail.
Plasma technology in the nuclear fuel cycle
The nuclear fuel cycle is schematically presented in Figure 1. The red arrows indicate where plasma technology can be used to replace conventional processes.
UF6 is a gas that is used for the enrichment of uranium. Processed uranium ore as received from the mines may consist of various chemical compounds, depending on the specific process that the mine uses. The conventional conversion route for UF6 production is a complex process involving multiple steps, each of which requires a separate, complete chemical plant. There are many minor variations on this process, but in general it can be described in as follows.
Uranium oxide, which can be UO3or U3O8, (also referred to as yellowcake) or ammonium diuranate (ADU or (NH4)2U2O7) is calcined to UO2 with hydrogen. The UO2 is hydrofluorinated with hydrofluoric acid (HF(g)) to form UF4, a solid green compound, which is then fluorinated with fluorine gas to form UF6(g). This process is schematically presented in Figure 2. Hydrofluoric acid is produced by the reaction of CaF2 with H2SO4 according to Equation [1]. F2 gas is produced by electrolysis of KF.HF.
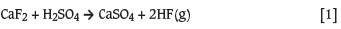
The AEC proved in concept that a uranium oxide could be directly fluorinated to UF6 with capacitive and inductive coupled non-thermal plasma according to Equation [2] (Jones, Barcza, and Curr, 1993). This will eliminate the multistep process presented in Figure 2 with a major economic advantage.
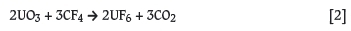
Uranium metal can also be fluorinated by F2 or CF4 or mixtures thereof in an inductive coupled non-thermal plasma (Equation [3]).

The AEC (now Necsa), demonstrated the use of a direct current (DC) plasma process to convert UF6 to UF4 using hydrogen or cracked ammonia as reductant. This process was scaled to a production rate of 1 kg UF4 per hour in a continuous operation. It was estimated that the process could be 30% more economical than the conventional process. The manufacture of depleted uranium metal from UF4 was also demonstrated, and a total of 13 kg of depleted uranium was manufactured using this process by the AEC using a scaled-up laboratory system. Processes have been proposed by Toumanov (2003) and Fridman (2008, p. 449) for the direct conversion of UF6 to uranium metal (Equation [4]).

This process is highly endothermic and takes place at temperatures above 5000 K. High quenching rates in the order of 10-7 seconds are required to prevent the reverse reaction from taking place.
Beta-UF5(s) is an unwanted reaction product that forms under certain conditions during the enrichment process. A high-frequency non-thermal plasma process was utilized for the in situ back-fluorination of beta-UF5 to UF6 using a mixture of CF4/argon or fluorine/argon.
Unfortunately, further developments regarding the abovementioned nuclear plasma processes were halted when South Africa's nuclear programme was terminated in 1993.
Nuclear nano-materials
Plasma technology is an extremely useful and effective technique for the manufacture of nanoparticles, especially with regard to advanced ceramics (Fridman, 2008, pp 417-498, 566; Van der Walt, 2012; Boulos, Fauchais and Pfender, 1994). A typical plasma system for the manufacturing of nanopowders is presented in Figure 3. The process involves the evaporation of reactants in the high-temperature environment of a plasma arc, followed by rapid quenching of the vapour in order to nucleate the particles. Ceramic powders such as carbides, nitrides and oxides have been synthesized in this way. Ceramic materials like SiC, ZrC, B4C and ZrO2, which are stable at high temperatures, have numerous applications in the nuclear industry, and will become even more prominent in Generation IV high-temperature gas-cooled nuclear reactors (HTGRs) (Konings et al., 2012; Kok, 2009).
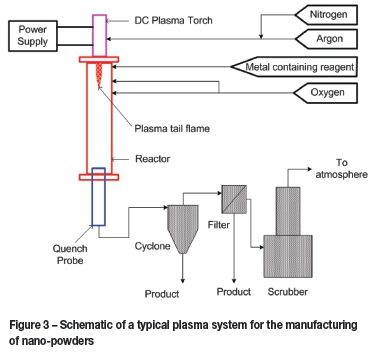
Necsa has produced several of these nanomaterials. Nano-zirconia can be produced by the plasma dissociation of zircon, followed by selective removal of the formed amorphous silica by fluorinating agents. Necsa commissioned and operated a pilot plant with a capacity of 100 kg/h to produce plasma-dissociated zircon and a 10 kg/h plant for the production of nano-silica.
Boron carbide is often applied in nuclear technology as a neutron shielding material and as control rods inside nuclear reactors. It has a high neutron absorbance cross-section for thermal neutrons of 755 barns and a high melting point of 2723 K (Kirk-Othmer Encyclopedia of Chemical Technology; Lipp, 1965). Necsa produced nuclear-grade B4C powder in a 30 kW non-transferred arc DC plasma system according to Equation [5]. BCl3 was evaporated at 60°C and fed into the plasma reactor at a rate of 120 litre per hour. The reaction took place at about 2200°C, and nitrogen was used as a quench gas. A particle size of between 80 nm and 100 nm was obtained, depending on the plasma parameters. Nano-sized B4C powder has enhanced compaction properties for the production of B4C pellets.
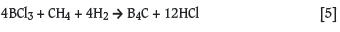
Nano-sized ZrC can also be manufactured by a plasma process.
Fluorocarbons in the nuclear industry
PTFE is one of the few fluorocarbons used in the nuclear industry. A sintered filter was produced from PTFE and used in the enrichment stages.
A thermal chemical process was developed to produce CF4 gas by reacting solid carbon with pure fluorine. A 99 % conversion was achieved, and the gas was used in a plasma process to produce PTFE monomer. The chemical formulae governing these reactions are presented in Equations [6] and [7]:
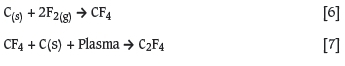
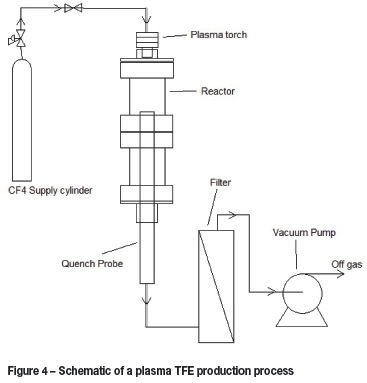
Various CF4 plasma plants for the production of TFE (C2F4) were constructed and commissioned at Necsa. A small 30 kW laboratory system and 100, 150, and 450 kW pilot systems were successfully developed and operated. A typical plasma TFE production system is presented in Figure 4.
As part of a DST-funded Fluorochemical Expansion Initiative, the C2F4 (TFE) was used in a suspension polymerization process to produce PTFE. After PTFE had been produced successfully, the second stage of the project to produce FEP was developed and proven. A schematic diagram of the system is presented in Figure 5. Since neither of these polymers are produced in South Africa, this an opportunity for a new business.
Plasma treatment of nuclear waste Vitrification
Vitrification is a recognized standard method of reducing the environmental impact of harmful waste. In vitrification, the solid component of the treated waste is encapsulated by high-temperature treatment. In recent years a glass formulation has been added to the vitrification process that allows the tailoring of the waste matrix. Nuclear waste containment in glass has several advantages: it guarantees the stability of the waste package over very long time periods (50-300 years) and could reduce the waste volume. Disposal safety criteria can be more easily met by adaptation of the waste matrix. This aspect is crucial in the treatment and disposal of nuclear waste due to the long periods of storage required. With the addition of a glass/ceramic mixture that encapsulates the waste, the resistance to leaching and chemical attack can be optimized (Petijean et al., 2002). The matrix developed for the containment of radionuclides should take into account the following issues (Luckscheiter and Nesovic, 1996; Bisset and van der Walt, 2009):
►The waste must be accommodated within the matrix composition
►Waste loading
►Characteristics such as resistance to solubility, phase separation, and devitrification
►Long-term behaviour such as thermal stability, irradiation resistance and chemical and mechanical stability
►Process considerations such as melting temperature, viscosity and electrical conductivity.
A thermal plasma is able to decompose various types of waste into a gas and a residue by exposing it to a very high temperature. Because of the very high temperature in the plasma, no sorting is necessary, thus human exposure is minimized. This system also has the capability of containing the uranium contamination in the molten pool of metal (if the waste is in a drum) as well as in ceramics and non-combustible materials.
The cost implications will include a capital installation cost and an operational cost. If designed properly, a plasma volume reduction system for nuclear waste can generate electricity to feed back into the process, reducing the operating cost to a minimum. There is a case to be made whether the long-term cost of disposal at a waste repository like Vaalputs will be comparable to the costs of volume reduction of nuclear waste by a plasma process. The additional benefits of the plasma system are the production of a vitrified product and the reduction of the waste volume (van der Walt and Rampersadh, 2011).
Uranium recovery
The recovery of valuable constituents such as uranium from historical and current waste streams is important and may also be economically viable. Plasma technology could replace other competing processes for this purpose, and may have important advantages over the conventional processes. Conceptual research was done on one such waste stream, namely the coated uranium kernels used in the pebble bed modular reactor (PBMR) project.
An alternative recovery method to the mechanical crushing of off-specification tri-structural-isotropic (TRISO) coated fuel microspheres was demonstrated. It was shown that the inert SiC layer can be completely removed by etching with the active fluorine species by an inductively coupled radio-frequency CF4 glow-discharge impinging a static bed from the top, at a working pressure of 1 kPa (van der Walt et al., 2011). The apparatus is shown in Figure 6.

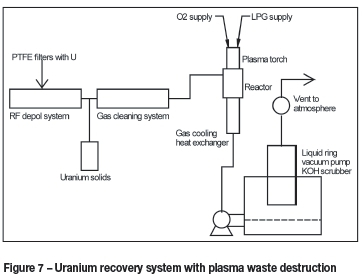
The recovery of uranium from contaminated PTFE filters was also investigated by Necsa. A process was developed that depolymerizes PTFE and thereby separates the matrix from the extremely valuable uranium. This system makes use of radio frequency (RF) induction heating to heat a reactor up to the depolymerization temperature of PTFE. Inside the reactor the PTFE is depolymerized and the product gas is cooled, separated from residual uranium particles, scrubbed and evacuated to a destruction facility where fluorocarbons are converted into CO2 and HF by means of a DC thermal plasma system using O2 and water gas. The HF is scrubbed by means of a KOH scrubber. A schematic diagram of this system is presented in Figure 7.
The AMI zirconium process
In 2005, the DST initiated the Advanced Metals Initiative (AMI) programme and asked Necsa to coordinate the New Metals Development Network, with the specific focus on the manufacturing of nuclear-grade zirconium metal from the mineral zircon (Zr(Hf)SiO4). The request from DST was specifically to develop a new and more economical way to manufacture Zr metal using Necsa's plasma and fluoro-chemical expertise and existing facilities. The conventional methods are described in detail in the scientific and patent literature dating back to the early 1920s (Blumenthal, 1958). Significant industrial manufacturing of nuclear-grade zirconium started in the late 1940s after World War II, when the advantages of using zirconium in nuclear power plants were realized. In general, most industrial manufacturing processes for nuclear-grade zirconium use high-temperature carbochlorination of zircon, selective separation of ZrCl4 and SiCl4, the separation of Zr and Hf by means of solvent extraction, distillation, selective crystallization, etc., and eventually the reduction of purified ZrCl4 with magnesium via the Kroll process (Equation [2]). These conventional processes can consist of between 16 and 18 individual steps, making the manufacturing of nuclear-grade zirconium one of the most expensive operations in the nuclear fuel cycle.
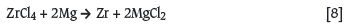
Zircon is an extremely chemical inert mineral. However, activating zircon with a plasma process to produce plasma-dissociated zircon (PDZ) makes it very reactive, especially towards fluoride-containing compounds such as HF and ammonium bifluoride (ABF, NH4F.HF) (Nel et al., 2011a, 2011b). Necsa operated a semi-commercial PDZ plant from 1995 to 2003, which had a capacity of 100 kg/h using a 3 χ 150 kW DC plasma torch configuration (Havenga and Nel, 2011). Necsa developed a plasma process for the manufacturing of Zr metal powder from PDZ, making use of the reactivity of PDZ with ABF to produce ZrF4 (Makhofane et al., 2011). ZrF4 or ZrCl4 is reduced with magnesium in a in a 30 kW DC non-transferred arc plasma plasma process (similar to the Kroll process) (Nel et al., 2012). The whole process is generally referred to as the AMI zirconium metal process (Nel et al., 2013). The plasma process has, however, the additional advantage that it can be modified to make it a continuous process, unlike the conventional Kroll process. The AMI zirconium metal process making use of plasma/fluoride technology consists of only six steps, and offers the potential of huge cost savings in comparison with the conventional processes.
This process is schematically presented in Figure 8.
Conclusions
Plasma processing has numerous potential applications in nuclear science and technology and for the manufacturing of nuclear materials. Over a period of three decades, Necsa has developed plasma applications in the nuclear fuel cycle, including the manufacture of nuclear ceramics and nanopar-ticles that are being (or can be) used in nuclear reactors, especially in high-temperature gas-cooled reactors. Fluoromonomers, which are used as precursors for many fluoropolymers, can also been made via plasma processes. Fluoropolymers are extensively used as filters, seals, and containers in the nuclear industry. Nuclear waste destruction can also be accomplished by plasma processes. Many of these processes have been developed to pilot scale, while others were developed only to the laboratory scale and proof-of-concept. Necsa has patented plasma and fluoride processes for the manufacturing of nuclear-grade zirconium metal powder using the mineral zircon as starting material.
Paper received Aug. 2015














