Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.10 Johannesburg Oct. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n10a6
Titanium and zirconium metal powder spheroidization by thermal plasma processes
H. Bissett; I.J. van der Walt; J.L. Havenga; J.T. Nel
The South African Nuclear Energy Corporation SOC Ltd., (Necsa)
SYNOPSIS
New technologies used to manufacture high-quality components, such as direct laser sintering, require spherical powders of a narrow particle size distribution as this affects the packing density and sintering mechanism. The powder also has to be chemically pure as impurities such as H, O, C, N, and S causes brittleness, influence metal properties such as tensile strength, hardness, and ductility, and also increase surface tension during processing.
Two new metal powder processes have been developed over the past few years. Necsa produces zirconium powders via a plasma process for use in the nuclear industry, and the CSIR produces titanium particles for use in the aerospace industry.
Spheroidization and densification of these metal powders require re-melting of irregular shaped particles at high temperature and solidifying the resulting droplets by rapid quenching. Spherical metal powders can be obtained by various energy-intensive methods such as atomization of molten metal at high temperatures or rotating electrode methods. Rapid heating and cooling, which prevents contamination of the powder by impurities, is, however, difficult when using these methods for high-melting-point metals. For this reason plasma methods should be considered.
Thermal plasmas, characterized by their extremely high temperatures (3000-10 000 K) and rapid heating and cooling rates (approx. 106 k/s) under oxidizing, reducing, or inert conditions, are suitable for spheroidization of metal powders with relatively high melting points. Thermal plasmas for this purpose can be produced by direct current (DC) plasma arc torches or radio frequency (RF) inductively coupled discharges. In order to obtain chemically pure spheroidized powder, plasma gases such as N2, H2, O2, and CH4 cannot be considered, while Ar, Ne, and He are suitable. Neon is, however, expensive, while helium ionizes easily and it is therefore difficult to obtain a thermal helium plasma at temperatures higher than 3000 K. Therefore argon should be used as plasma gas. Residence times of particles in the plasma region range from 5-20 ms, but this is usually sufficient as 7-8 ms is required for heating and melting of titanium or zirconium metal particles in the 30 μm size range at 3500 K. In this study the melting and spheriodization of titanium powders was investigated by DC non-transferred arc and RF induction plasma methods. The powders were characterized before and after plasma treatment by optical microscopy and scanning electron microscopy (SEM) to observe if any melting or spheroidization had occurred.
Keywords: plasma, zirconium, titanium, spheroidization.
Introduction
Contemporary product design processes have been affected by new technologies that assist the manufacturing sector to meet the specifications of specialized components used in the aerospace and medical industries (Williams and Revington, 2010). Direct selective laser sintering (SLS) or, more specifically when considering the processing of metal, direct metal laser sintering (DMLS), are some of these technologies. Implants used in the medical industry to repair or replace bone structure must be strong, ductile, and biocompatible. Materials used for this purpose are stainless steel, cobalt, chromium alloys, or pure titanium, with titanium (Ti) being the material of choice (Taylor, 2004; Bertol et al., 2010). Titanium is also used for applications in the aerospace industry due to its light weight, excellent corrosion resistance, high strength, and attractive fracture behaviour (Li et al., 1997). In order to manufacture a high-quality component such as an implant, the titanium powder used as feed material should be dense and spherical. Powder particle size can affect the material spreading and the sintering rate due to the fact that the shape, density, and size of the particles have an effect on their packing density, sintering mechanism, and the flowability of the powder during feeding (Gignard, 1998, p. 34; Despa et al., 2011).
The chemical purity of the titanium powder during processing is also very important. Surface oxidation should be prevented as this increases the surface tension, hindering material from flowing during sintering. Oxidation also results in poor bonding between sintered lines affecting the manufactured structures, while nitrides reduce the material's corrosion resistance (Gignard, 1998, p.34).
South Africa has an opportunity to gain benefit from its abundant titanium-bearing mineral reserves through value beneficiation. The potential applications and markets for titanium are in aerospace, the armaments industries, naval applications, offshore oil industries, architecture, and medicine. By establishing a local titanium industry in South Africa, enterprise development across all segments is expected (Van Vuuren, 2009).
A convenient way to produce spherical particles, and in particular titanium particles, is to re melt irregularly shaped titanium particles at high temperatures and solidify the droplets by rapid quenching. This can be done by using thermal plasmas, which are characterized by their extremely high temperatures (3 000-10 000 K) and rapid heating and cooling rates (approx. 106 K/s) under oxidizing, reducing, or inert conditions. Plasma is a partially or fully ionized gas in a condition of quasi-neutrality. Thermal plasmas used for particle spheroidization are generally produced by devices such as direct current (DC) arc plasma torches and radio frequency (RF) inductively coupled discharges. Thermal plasmas with temperatures as high as 2 χ 104 K can be obtained (Li and Ishigaki, 2001).
This paper presents a short background on the DC plasma and RF induction plasma systems used for spheroidization, as well as some background on the properties of powder before and after plasma treatment. A few experimental results relating to the spheroidisation of titanium powder using DC plasmas and RF induction plasmas at the South African Nuclear Energy Corporation (Necsa) are discussed.
Thermal plasmas for spheroidization
Although various plasma methods can be used, RF induction plasmas are the preferred method for the spheroidization and densification of particles. This is due to the longer residence time in the plasma and also the lower possibility of contamination caused by electrode erosion. The residence times commonly employed for particle melting in RF plasmas range from 5 to 20 ms (Gignard. 1998, p. 24). DC arc plasma torches, although not regularly used for spheroidization, are also an option. Necsa has expertise in the design and operation of these plasma torches. A short description of the RF and DC plasma torch methods is given, but the complete system, including as particle feeding, quenching, and collection methods, will not be discussed. Information on the spheroidization of titanium metal powders by thermal plasmas has not been found in the public domain, and literature available on spheroidization by DC thermal plasmas is limited to in house designed plasma torches.
RF induction plasma
In an induction torch, the energy coupling between the electric generator and the plasma itself is done by a cylindrical coil (Gignard, 1998, p. 6). A typical induction setup is illustrated in Figure 1.
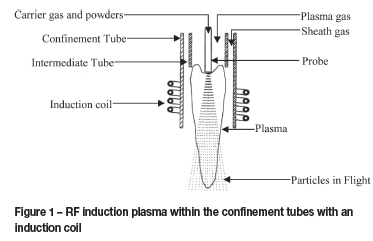
The 'flame' properties for a RF induction plasma are dependent on the power input, frequency, and the gas pressure and composition. Less power is required at higher frequencies and lower gas pressures to maintain a plasma. Comparing argon and hydrogen, less power is required to maintain an argon plasma due to the fact that argon ionizes more easily than hydrogen (Gignard, 1998, p. 9), while higher plasma gas temperatures can be obtained at higher operating pressures.
Although various RF induction plasma systems are available, several research studies have utilized those available from Tekna Systems Incorporated. Most of these studies have used the Tekna PL 50 torch, which can be operated at various frequencies (0.3, 2, or 3 MHz) and power inputs (30, 40, 50 and 100 kW). For spheroidization, Ar or Ar/H2 is used as plasma gas (Jiang and Boulos, 2006; Li and Ishigaki, 2001; Gignard, 1998, pp. 35-42). The use of the Tekna PL 035LS torch has also been reported (Károly and Szépvölgyi, 2005).
DC plasma
There are two types of DC torches, these being non-transferred and transferred arc torches. In non-transferred arc DC torches, the electrodes between which the arc is created are inside the body of the torch itself. The plasma gas moves through the arc and is ionized to form the plasma jet. In Figure 2 a typical configuration for a non-transferred arc plasma torch is shown.
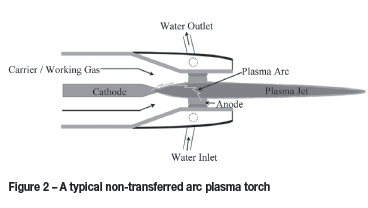
Non-transferred arc plasma torches are also used in plasma spraying. In this method a powder is introduced into the plasma jet. In this instance, however, spherical powders with a narrow size distribution are used to spray dense, even coatings onto substrates. The purpose of this method is not to spheroidize the powder during the process, but the principle remains similar to spheroidization. In both instances the powder will be quickly melted followed by rapid quenching.
Powder properties
Properties such as particle size, bulk density, morphology, impurities levels, etc. are pivotal in selecting a suitable powder to be used in SLS. Particle size affects the material spreading and the sintering rate. The morphology, density, and size of the particles have an effect on their packing density, sintering mechanism, and the flowability of the powder during feeding (Gignard, 1998, p. 34; Despa and Gheorghe, 2011). Thermal treatment of a powder results in spherical particles with an increased bulk density and decreased in impurity levels, while improving the flowability of the powder.
Density and flowability of powders
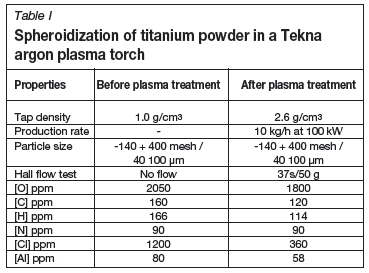
Bulk density refers to the density of an uncompacted mass of powder taking into account the interparticular voids. The tap density refers to density of a compacted mass of powder where efforts have been made to eliminate the interparticular voids by repeated tapping of the powder. A standardized Hall flow method is used to investigate the apparent density and flow characteristics of free-flowing metal powders (ASTM. 2006). In Table I the increases in tap density and Hall flow rate are indicated for titanium powder treated by a Tekna torch operated at 50 kW (Boulos et al., 2011).
Impurities levels
Titanium becomes brittle due to its affinity for oxygen, nitrogen, and hydrogen. Contamination of titanium by air (more specifically oxygen) and hydrogen is thus a problem during welding or sintering. This contamination causes an increase in tensile strength and hardness but reduces ductility, resulting in crack formation. For welding, 0.3% oxygen, 0.15% nitrogen, and 150 ppm hydrogen are seen as the maximum tolerable limits. Surface discolouration gives a good indication of the degree of atmospheric contamination. The colour of the metal changes from silver to a light straw colour (shades of yellow), then dark straw, dark blue, light blue, grey and finally white (TiO2) as contamination increases. The light and dark straw colours indicate light contamination, which is usually acceptable. Dark blue indicates more contamination, while light blue, grey and white indicate high levels of contamination (TWI, 2014).
Impurity levels of elements such as O, C, H, N, and S can be determined by using combustion methods or instrumental gas analysis (IGA).
In Table I the decrease in impurity levels is clearly indicated for titanium powder treated by a Tekna torch operated at 50 kW using an argon plasma.
Visual characterization of powders
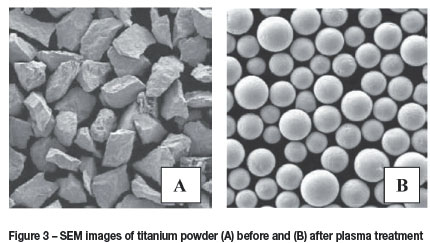
Particle morphology can be investigated by using optical microscopy or scanning electron microscopy (SEM) to determine whether melting or spheroidization of the powder occurred during thermal plasma treatment. Figure 3 shows SEM images of titanium powder before and after treatment by a Tekna torch operated at 50 kW using an argon plasma. The images clearly indicate that the rapid heating followed by cooling resulted in spheroidization of the particles.
Experimental
The titanium powder used in this study was obtained from the Council for Scientific and Industrial Research (CSIR). Due to the limited quantity of powder available, the as received powder was characterized using SEM and an in-house manufactured Hall flow meter according to ASTM (ASTM. 2014). The amount of powder available for experimentation was not sufficient for post-plasma measurement of the flowability using the Hall flow funnel, and for this reason, SEM and optical microscope images of the powder before and after plasma treatment were used to assess the success of the spheroidization. Similarly, no assessment of the impurity levels of the powder before and after plasma treatment was possible.
As-receved powder characterization
The as-received and plasma-treated powders were characterized by SEM using a Quanta FEI 200 D instrument. Optical images of the powders were also obtained where possible. A Zeiss Discovery V20 stereo microscope was used for this purpose, and the images recorded utilizing Zeiss Axiovision software.
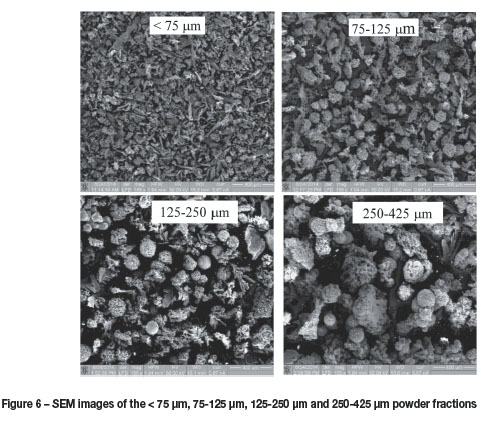
Owing to the large variation in the sizes of the particles observed, the as-received powder was separated into four fractions using sieve mesh sizes of 75, 125, 250, and 425 pm. The four fractions were classified as < 75 pm, 75-125 pm, 125-250 pm and 250-425 pm.
Spheroidization by thermal plasmas
Two high-temperature plasma systems were available for the experiments, namely a RF induction plasma and a non-transferred arc DC plasma.
RF thermal plasma system
The RF plasma system is shown in Figure 4. This system was used for conceptual work and therefore the exact conditions (gas flow rates, powder flow rates, and input power) of the experiments are not given at this stage. The RF induction coil was connected to a 3 MHz power supply with a maximum power input of 10 kW. Inside the induction coil, two quartz tubes of different diameters were mounted in such a manner as to allow deionized cooling water to flow between them in order to cool of the quartz tube. Above the quartz tube, a hopper loaded with titanium powder was mounted and connected to a 1 mm orifice. Once the system was leak-tight, the system was evacuated and a non-thermal plasma-initiated. Argon gas was slowly introduced. The plasma was gradually changed from a non-thermal to a thermal plasma by increasing the operating pressure (increasing argon flow) and input power until a stable thermal plasma was maintained. At this stage the titanium powder was fed through the thermal plasma. Once a sufficient amount of a given powder fraction had been treated, the plasma was extinguished and the reactor allowed to cool. The powder was then removed for imaging by SEM and optical microscopy. The temperature of the plasma gas was not estimated due to the complexity of the temperature determination method. All four powder fractions were treated.
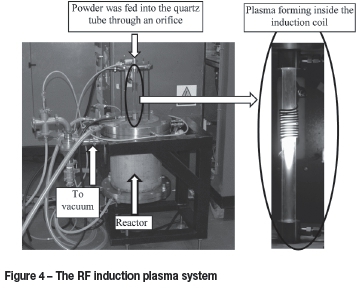
DC thermal plasma system
A schematic of the non-transferred arc DC plasma system is shown in Figure 2. The system utilized a 30 kW DC power supply with a maximum current input of 150 A. The system was operated at pressures slightly higher than atmospheric. In order to obtain temperatures high enough for spheroidization of titanium (approx. 3300 K) the system was operated using a nitrogen plasma. The reason for this is that argon or helium ionize easily and therefore the voltage (resistance) cannot be increased sufficiently. In order to obtain high enough temperatures using argon or helium, the power supply would need to be operated at currents between 400 and 600 A, which fall outside the window of operation of the power supply used in this study. The plasma torch was mounted in a water-cooled reactor and operated at 150 A and 200 V. The plasma gas was fed through the torch at a rate of 1.43 g/s. The plasma gas temperature was estimated by calculating the plasma gas enthalpy (kJ/kg) and relating this to temperature. Once the plasma was stable, the as received powder was fed near the tail flame (plasma jet) of the plasma at a rate of 5 g/min.
Results and discussion
As-received powder characterization
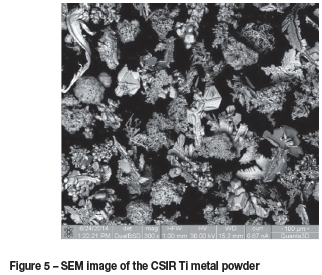
Figure 5 shows an SEM image of the as-received powder. It is clear that the powder consisted of various particles sizes and morphologies. Some particles appeared to be crystalline while others appeared amorphous. The particles had rough needlelike structures, porous round structures, and porous irregular structures.
The SEM images of the four size fractions of the powder are shown in Figure 6. Even after size classification, large variations in particle morphology and structure were observed.
RF thermal plasma treatment
The temperature of the RF thermal argon plasma could not be calculated or determined at this stage. It is estimated that the temperature of the plasma was near 3300 K (enthalpy of 1.6 MJ/kg for argon) due to the fact that particle melting was achieved.
Figure 7 shows an SEM micrograph of the as-received powder after RF thermal plasma treatment. It can be seen that the smaller particles were affected by the thermal treatment. Some spherical particles were observed, as indicated by the arrows.
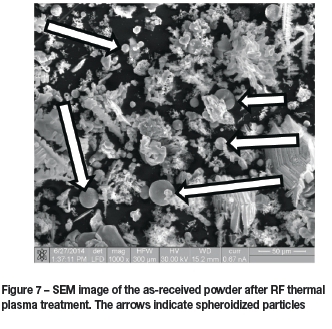
Figure 8 shows a SEM image of the < 75 pm powder fraction after plasma treatment. In most instances it appeared as though spherical particles smaller than 150 pm were obtained, although some agglomeration occurred resulting in large irregularly-shaped particles.
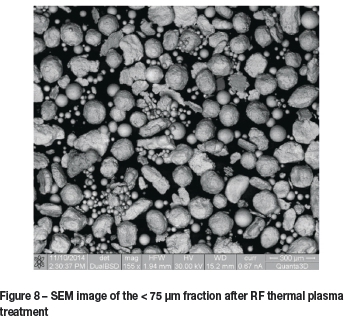
Similar results were obtained using the 75-125 μm fraction, although slightly fewer spherical particles were observed. The large fractions (125-250 μm and 250-425 μm) were not affected by the plasma treatment. These results suggest that particles smaller than 125 μm can be spheroidized. Agglomeration of melted particles is a concern, however, and for this reason a sheath gas is required. Agglomerate formation is possibly due to the collection of fine particles near the quartz tube. The particles 'roll' down the tube surface, forming relatively large agglomerates. The sheath gas is not only necessary to prevent agglomeration from occurring, but will also prevent the collection of fine particles near the coil region of the quartz tube, a regularly observed occurrence. Once titanium metal particles collect on the surface of the quartz tube, induction heating of the particles occurs, resulting in a reduction of the plasma gas temperature.
DC thermal plasma treatment
The DC plasma torch efficiency was calculated to be 61%. Therefore 18.3 kW of the rated 30 kW was available for the plasma gas, which at a nitrogen gas feed rate of 1.43 g/s relates to a gas enthalpy of 12.8 MJ/kg for nitrogen. From thermodynamic data it is estimated that the gas temperature near the arc was approximately 6200 K. Further from the arc the temperature was approximately 3300 K (enthalpy of 3.7 MJ/kg for nitrogen). For the DC plasma treatment, only the as-received powder was used and not the separate fractions. The estimated enthalpy cost per unit mass titanium for spheroidization was 60 kWh/kg (216 MJ/kg).
Optical micrographs of the treated powder are shown in Figure 9. A high degree of spheroidization is evident. Due to the fact that nitrogen was used as plasma gas, surface contamination occurred, which is evident from the colours observed in the images. The light and dark straw colours indicate light contamination, which is usually acceptable. Dark blue indicates more contamination, while light blue indicate high levels of contamination (TWI, 2014).

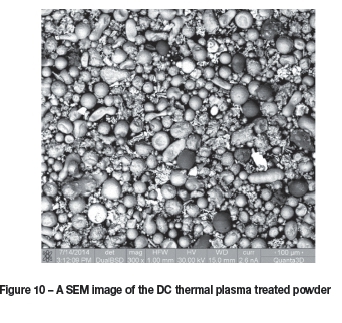
A SEM image of the treated powder is shown in Figure 10. The variation in the contrast of the particles indicates various degrees of contamination by either nitrogen or oxygen. Again, a high degree of spheroidization is evident. A nitrogen plasma was used in this instance, and it is expected that an argon plasma will yield similar results with respect to spheroidization of the particles, but without surface contami nation occurring.
Conclusions
Available literature indicated that titanium metal particles can be spheroidized in a thermal plasma by re melting of irregularly shaped particles at high temperatures and solidifying the droplets by a rapid quench. Thermal plasma treatment of powders results in improved powder flow characteristics and an increased density of individual particles, pivotal in selecting a suitable powder to be used in SLS or DMLS.
The titanium powder received from the CSIR consisted of various particle sizes and morphologies, where some particles appeared to be crystalline and others amorphous.
In this study SEM images indicated that the powder could be spheroidized by an RF thermal plasma using argon gas without increasing impurity levels significantly. Various powder fractions were tested. Only the < 75 μm and 75-125 μm fractions could be spheroidized; the larger fractions (125250 μm and 250-425 μm) were not affected by the plasma treatment.
SEM and optical microscope images also showed that titanium powder could be effectively spheroidized by a DC thermal plasma using nitrogen gas. In this instance the powder was used as-received and not sieved into fractions. The particles changed colour, indicating surface contamination by nitrogen. It is, however, expected that spheroidization without contamination will be possible by DC thermal plasma treatment using argon gas at a gas temperature near 6200 K.
References
ASTM International. 2006. Standard test method for apparent density of free-flowing metal powders using the Hall flowmeter funnel. Designation: B212 99. West Conshohocken, PA. [ Links ]
Bertol, L.S., Junior, W.K., Da Silva, F.P., and Aumund, K.C. 2010. Technical report Medical design: Direct metal laser sintering of Ti 6Al 4V. Materials and Design, vol. 31. pp. 3982 - 3988. [ Links ]
Boulos, M., Heberlein, J., and Fauchais, P. 2011. Thermal plasma processes, fundamentals and applications. Short course presented at the university of Pretoria, 28 29 October. 2011. Pretoria, South Africa. [ Links ]
Despa, V. and Gheorghe, I.G. 2011. Study of selective laser sintering: a qualitative and objective approach. Scientific Bulletin of Valahia University Materials and Mechanics, vol. 6. pp. 150 - 155. [ Links ]
Gignard, N.M. 1998. Experimental optimization of the spheroidization of metallic and ceramic powders with induction plasma. Thesis, National Library of Canada, Sherbrooke, Quebec, Canada. [ Links ] .
Jiang, X. and Boulos, M. 2006. Induction plasma spheroidization of tungsten and molybdenum powder. Transactions of Nonferrous Metal Society of China, vol. 16. pp. 13 - 17. [ Links ]
Li, Z. Gobbi, S.L. Norris, I. Zolotvsky, S., and Richter, K.H. 1997. Laser welding techniques for titanium alloy sheet. Journal of Materials Processing Technology, vol. 65. pp. 203 - 208. [ Links ]
Li, Y. and Ishigaki, T. 2001. Spheroidization of titanium carbide powders by induction thermal plasma processing. Journal of the American Ceramic Society, vol. 84, no. 9. pp. 1929 - 1936. [ Links ]
Taylor, C.M. 2004. Direct laser sintering of stainless steels: thermal experiments and numerical modelling. PhD thesis, School of Mechanical Engineering, university of Leeds, uK. Chapter 2, p. 5. [ Links ]
TWI. http://www.twi-global.com/technical-knowledge/job-knowledge/welding-of-titanium-and-its-alloys-part-1-109 [Accessed 18 July 2014]. [ Links ]
Van Vuuren, D.S. 2009. Titanium - an opportunity and challenge for South Africa. Keynote address: 7th International Heavy Minerals Conference 'What Next', Champagne Sports Resort, Drakensberg, South Africa, 20-23 September 2009. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 1 - 8. [ Links ]
Williams, J.V. and Revington, P.J. 2010. Novel use of an aerospace selective laser sintering machine for rapid prototyping of an orbital blowout fracture. International Association of Oral and Maxillofacial Surgeons, vol. 39. pp.182 - 184. [ Links ]
Paper received Aug. 2015
Revised paper received Aug. 2015.














