Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.10 Johannesburg Oct. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115n10a5
Fluorine: A key enabling element in the nuclear fuel cycle
P.L. Crouse
Fluoro-Materials Group, Department of Chemical Engineering, University of Pretoria
SYNOPSIS
Fluorine - in the form of hydrofluoric acid, anhydrous hydrogen fluoride, elemental gaseous fluorine, fluoropolymers, volatile inorganic fluorides, and more - has played, and still plays, a major role in the nuclear industry. In order to enrich uranium, the metal has to be in the gaseous state. While more exotic methods are known, the standard and most cost-competitive way of achieving this is by means of uranium hexafluoride (UF6). This compound sublimates at low temperatures, and the vapour is enriched using centrifugal processes. The industrial preparation of uranium hexafluoride requires both elemental fluorine gas and anhydrous hydrogen fluoride (HF). HF is prepared by the reaction of sulphuric acid with fluorspar (CaF2). Fluorine gas in turn is prepared by the electrolysis of HF. Yellowcake is first converted to uranium tetrafluoride (UF4), using HF, after which the compound is treated with fluorine to yield UF6. After enrichment, the UF6 is reduced to UO2 for use in fuel elements in pellet form.
South Africa has the largest reserves of fluorspar internationally, and is the third largest producer after Mexico and China. Fluorine technology has many associated difficulties, because of the reactivity of fluorine and the toxicity of HF. The main barriers to entry into the fluorochemical industry are thus the abilities to produce both HF and F2. Both these substances are produced locally, at the industrial scale, at Pelchem SOC Ltd. Should South Africa contemplate developing its own nuclear fuel cycle as part of the awaited new-build nuclear project, it will be imperative to leverage the existing skills with respect to fluorine technology, resident at both Pelchem and Necsa, for this purpose.
This paper summarizes the fluorochemical skills developed locally over the past several decades, and suggests strategies for maintaining the technology base and developing it for the next generation of scientists and engineers.
Keywords: flourine, hydrogen flouride, nuclear fuel cycle.
Introduction
Fluorine chemistry has always had a very close relationship with the nuclear industry. Although the first mention of fluorspar was recorded in the sixteenth century, and the fundamentals had been well-developed by the Second World War, it was only during the Manhattan Project (Banks et al., 1994), when almost unlimited funds were made available for the large-scale industrialization of fluorine and related compounds, that the technology took off. Expertise in both the chemistry and the engineering aspects of fluorine is essential for the design and running of several of the unit processes in the nuclear fuel cycle.
The aim of this paper is to give a brief overview of the role of fluorine in the nuclear fuel cycle, and to outline the current South African fluorochemical capability.
Hydrogen fluoride and elemental fluorine
The major barrier to entry into the fluoro-chemical industry is the ability to manufacture and, in general, to handle fluorine and its precursor, hydrogen fluoride. Both are extraordinarily difficult and dangerous substances with which to work.
Hydrogen fluoride (HF) is produced by the reaction of fluorspar (calcium difluoride) with sulphuric acid:

The reaction is endothermic and reactors are generally run at temperatures above 200°C. HF is a clear liquid, with a boiling point of 19.6°C. It readily dissolves in water and, in its aqueous form, is known as hydrofluoric acid. Unlike the other common mineral acids, it is weak acid, thus does not readily deprotonate. Hydrofluoric acid is distinguished by its ability to dissolve glass, and as a consequence cannot be used in ordinary laboratory glassware.
Both hydrogen fluoride and hydrofluoric acid are enormously hazardous substances (Bertolini, 1992; Smith, 2004). Upon contact, human skin is not immediately burnt by the action of the hydronium ion; rather, because of the small size of the HF molecule, it diffuses through the skin and precipitates and inactivates biological calcium and magnesium subcutaneously, causing tissue necrosis. The wounds are extremely painful, and difficult to treat. In general the dead flesh has to be surgically removed, and a calcium gluconate solution injected into the tissue underneath the wound to prevent deeper diffusion of the HF. A typical HF wound is shown in Figure 1.
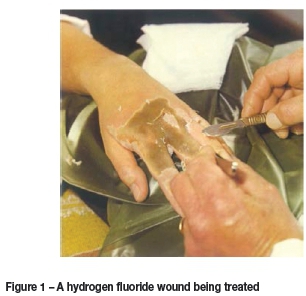
HF is also very corrosive, and materials selection is critically important in the development of an HF-related industrial process.
Although HF was first synthesized in the eighteenth century, and was known to contain an element, it was only in the late nineteenth century that fluorine was first isolated. This was accomplished by Henri Moissan (Argawal, 2007). Because it is a weak acid, anhydrous HF (AHF) does not conduct electrical current. Moissan's discovery was that the molten salt KF.xHF does indeed conduct electrical current and can be electrolysed. As a melt, e.g. with x=2, it dissociates and forms the equilibrium:

Moissan (Figure 2) was awarded the Nobel Prize in Chemistry in 1906. Since the first isolation of fluorine, the electrolysis process has undergone a few technical changes, but has remained more or less static throughout the past few decades. Comprehensive descriptions of the technology can be found in Slesser and Schram (1951), Rudge (1971), and Shia (2004).

Fluorine itself is the most reactive element in the periodic table, and reacts with all other elements, excluding only the two noble gases helium and neon (Cotton et al., 2007). In general the reactions of fluorine are highly exothermic, and because of its reactivity, materials of construction are of critical importance for safe operation. In general, expensive nickel-containing alloys are required. It should be noted that HF sells for US$1-3 per kilogram, while F2 sells for US$15-20 per kilogram. The high cost of fluorine can be attributed to electrical requirements and the high maintenance costs of the electrolysis cells.
South Africa is richly endowed with fluorspar (Roskill, 2009). Relative production and reserve figures are given in Table I. At present South Africa is the third largest producer of the ore, and has the largest reserves. China and Mexico, being closer to the larger international markets, are the two top producers.
A brief history of fluorine chemistry and technology (Ameduri, 2011)
A timeline for the major discoveries and developments in fluorine chemistry is listed below.
►Georg Bauer first describes the use of fluorspar (CaF2) as a flux in 1530 - as a flux aiding the smelting of ores by German miners
►Heinrich Schwanhard finds, in 1670, that fluorspar dissolved in acid and the solution could be used to etch glass
► From the 1720s, the effect on glass by adding sulphuric acid to fluorspar is studied
►Scheele (a Swedish scientist) 'discovers' fluoric acid (HF) in 1771
►Several chemists try unsuccessfully to isolate fluorine, and several die of HF poisoning during separation experiments
►The French chemist Moissan is the first to isolate elemental fluorine gas. He is awarded the Nobel Prize in 1906
►Swarts discovers the Cl/F exchange chemistry of SbF3
►Midgley discovers Freons in 1928
►In the 1930s General Motors begins using Freon-type fluorocarbons (CFCs) as replacement for hazardous materials e.g. NH3. CFCs also finds use in propellants and fire extinguishers
►1938 Plunkett of DuPont discovers Teflon®
►WW II and uranium enrichment
►In 1947 Fowler discovers the CoF3 method of perfluorination
►1949 sees Simons' discovery of electrochemical fluorination
►In the mid-1950s 3M invents ScotchguardTM
►Fried's initial pioneering work in 'medicinal fluorine chemistry' commences in 1954
►Neil Bartlett's discovery of noble gas chemistry in 1962 (XePtFa)
►Rowland and Molina's model for ozone depletion is published in 1974
►Hargreave's 'direct' perfluorination discoveries in 1979
►Fluorocarbon gases start finding application in the semiconductor industry in late 1980s
►2003 sees O'Hagan's isolation of first fluorinating enzyme
►Fluorine has become ubiquitous in pharmaceuticals, and is essential in medicinal chemistry.
At present there are more than 50 industrial producers of HF (Roskill, 2009), the source precursor for all industrial fluorochemicals. Because of cost considerations, it is always preferable in practice to employ a synthesis route that uses HF rather than F2.
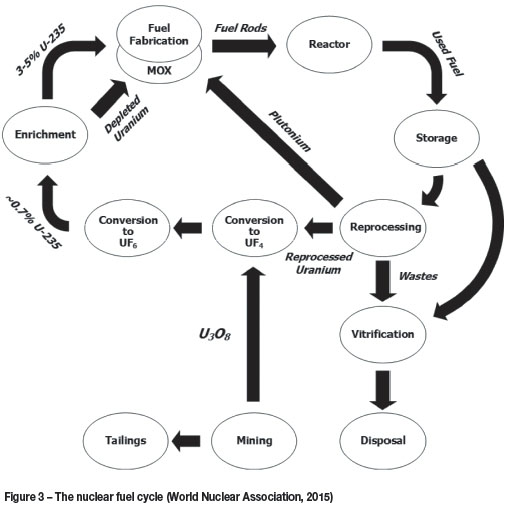
The nuclear fuel cycle
As indicated in Figure 3, fluorine plays a critical role in several of the unit processes in the nuclear fuel cycle. Generally, the uranium arrives at the conversion plant in the form of U3O8. In order for isotope separation to be effected, uranium is required in the form of a gaseous compound. This compound is UF6. U3O8 is converted to UF6 in a three-step process, each requiring its own plant. The oxide is first converted to UO2 in a hydrogen atmosphere, according to the reaction
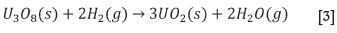
Note that U3O8 is a mixed valence oxide, thus reduction of a single U+6 to U+4 takes place. Subsequent to this, the uranium dioxide is converted into uranium tetrafluoride in the substitution reaction:
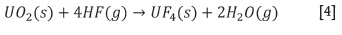
Finally, the uranium tetrafluoride is fluorinated to the hexafluoride, using elemental fluorine gas:
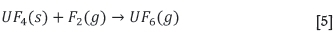
Enrichment now takes place, with fissionable U235separated from U238. This is normally done by centrifuge technology. The enriched uranium is used as solid uranium dioxide. There is more than one way of carrying out the reduction. A standard method is in a hydrogen-fluorine flame reactor. The high temperature is needed to initiate the reaction, given as
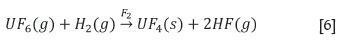
Solid uranium dioxide powder is pressed into pellets which are housed in Zircalloy tubes. These are bundled into fuel elements (in the case of pressurized-water reactors) ready for use.
UF6 is itself a powerful oxidant and fluorinating gas. Materials of construction for plants handling UF6 are thus similar to those for plants that have fluorine as reactant or product. Seals, filters, bearings, etc., are machined from various fluoropolymers, predominantly polytetrafluoroethylene (PTFE). Being fully fluorinated, PTFE is resistant to attack by fluorinating agents (Drobny, 2009; Ebnesajjad, 2013).
For comprehensive information about the nuclear fuel cycle, the reader is referred to Barré and Bauquis (2007), Kok (2009), Konings (2012), Tsoulfanidis (1996), Wilson (1996), and Yemelyanov (2011).
South Africa's fluorochemical capability
Highlights in the history of South African fluorochemical technology platform (Naidoo, 2015) are listed below.
►Nuclear conversion starts at the Atomic Energy Corporation (AEC) (now the South African Nuclear Energy Corporation, Necsa) in the 1960s
►Anhydrous hydrogen fluoride (AHF) and fluorine (F2) are required for uranium hexafluoride (UF6) production. AECI acquires the technology in the 1970s
►AECI stops producing AHF in 1984
►Necsa commissions an HF plant in 1985
►Industrialization and commercialization - 1992
►The Fluorochemical Expansion Initiative (FEI) is adopted as a national initiative - 2006
►Pelchem mandated to champion FEI by Necsa Board of Directors - 2007
►Two research chairs are founded via the National Research Foundation's SA Research Chairs Initiative (SARChI), one at the University of KwaZulu-Natal (UKZN) and one at the University of Pretoria (UP).
At present Necsa and its wholly-owned subsidiary Pelchem SOC Ltd are the main centres of South African fluorochemical expertise, along with two university chairs, one at the University of KwaZulu-Natal (UKZN) and the other at the University of Pretoria (UP). Pelchem runs a commercial 5000 t/a HF plant. Figure 4 shows a photograph of the rotary kiln HF reactor.

The company also operates some 20 fluorine electrolysis cells (Figure 5).

Pelchem supplies a range of fluorine products to the local and international markets. These include xenon difluoride, nitrogen trifluoride, various organofluorine compounds, perfluorinated alkanes, and a variety of inorganic fluoride salts.
Although a full fuel cycle existed on the Pelindaba site, it was abandoned in 1995. The technology in effect does not exist anymore, and if a new fuel cycle is to be established in South Africa, it will have to be a start-up from scratch rather than resuscitation of the old technology. Should this come to pass, our fluorochemical expertise, both existing and under development, will be invaluable if not essential. This will be the case whether the conversion is purchased off the shelf or developed locally. The operation of a conversion plant requires detailed and extensive fluorochemical expertise.
The road ahead
Since the inception of the Fluorochemical Expansion Initiative (FEI), South Africa has made considerable inroads into the development of its fluorochemical capability. The Necsa research effort has been strengthened and expanded, the SARChI Chair at UKZN has been extremely productive regarding research into various thermodynamic aspects of industrial fluorochemical processes, UP is developing a fluoropolymer capability, and Pelchem SOC Ltd has commissioned a new pilot plant, known as the Multipurpose Fluorination Pilot Plant (MFPP). The next few years are critical. A number of things need to happen for the South African research and development effort to continue progressing, and for the technology to be leveraged for the nuclear build plan. These are:
►The next phase of FEI needs to be commercially successful, with visible new products
►The new cohort of scientists and engineers, trained via funding by FEI, SARChI, and the Advanced Metals Initiative (AMI), have to find employment in the fluorine/nuclear industry
►The decision about the nuclear new-build programme has to be taken sooner, rather than later. Within the next 5-7 years the majority of the last generation of Necsa senior scientists and engineers will have retired
►The current postgraduate training programme has to be accelerated, with Necsa senior scientists retained for co-supervision of dissertations and theses.
Acknowledgements
The author acknowledges the South African National Research Foundation for financial support via the SARChI programme, and Department of Science and Technology for funding through their Fluorochemical Expansion Initiative. Rajen Naidoo, current acting CEO of Pelchem, is thanked the plant photographs, and Dr Johann Nel and Gerard Puts are acknowledged for comment and assistance with graphics, respectively.
References
Agarwal, A. 2004. Nobel Prize Winners in Chemistry (1901-2002). APH Publishing,, New Delhi, India. [ Links ]
Ameduri, B. 2011. History of fluorine chemistry. Personal communication. [ Links ]
Banks, R.E., Samrt, B.E., and Tatlow, J.C. (eds). 1994. Organofluorine Chemistry; Principles and Commercial Applications. Springer Science, New York. [ Links ]
Barré, B. and Bauquis, P.R. 2007. Nuclear Power: Understanding the Future. Ronald Hirlé, Strabourg and Paris. [ Links ]
Bertolini, J.C. 1992. Hydrofluoric acid: A review of toxicity. Journal of Emergency Medicine, vol. 10, no. 2. pp. 163-168. [ Links ]
Cotton, F.A., Wilkinson, G., Murillo, C.A., and Bochmann, M. 2007. Advanced Inorganic Chemistry, wiley, New Delhi, India. [ Links ]
Drobny, J.G. 2009. The Technology of Fluoropolymers, CRC Press, Boca Raton, FL. [ Links ]
Ebnesajjad, S. 2013. Introduction to Fluoropolymers: Materials, Properties, Applications. Elsevier, Amsterdam. [ Links ]
Kok, K.D. 2009. Nuclear Engineering Handbook. CRC Press, Boca Raton, FL. [ Links ]
Konings, J.M. 2012. Comprehensive Nuclear Materials Vol 2. Elsevier, Amsterdam. [ Links ]
Naidoo, R. 2015. History of fluorine and HF at Necsa. Personal communication. Pelchem SoC Ltd. [ Links ]
Roskill Information Services. 2009. The Economics of Fluorspar. London. [ Links ]
Rudge, A.T. 1971. Preparation of elemental fluorine by electrolysis, Introduction to Electrochemical Processes. Kuhn, T. (ed.). Elsevier, Amsterdam. Chapter 5. [ Links ]
Shia, G. 2004. Fluorine. Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ. [ Links ]
Slesser, C. and Schram, S.B. (eds). 1951. Preparation, Properties, and Technology of Fluorine and Organic Fluoro Compounds. McGraw-Hill, New York. [ Links ]
Smith, R.A. 2004. Hydrogen fluoride. Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ. [ Links ]
Tsoulfanidis, N. 2010. The Nuclear Fuel Cycle. American Nuclear Society, Scientific Publications, La Grange Park, IL. [ Links ]
Wilson, P.D. 1996. The Nuclear Fuel Cycle: From Ore to Waste. Oxford Scientific Publications, oxford. [ Links ]
World Nuclear Association. 2015. The nuclear fuel cycle.. http://www.world-nuclear.org/info/Nuclear-Fuel-Cycle/ [Accessed July 2015]. [ Links ]
Yemelyanov, V.S. and Yesvstyukhen, A.I. 2011. The Metallurgy of Nuclear Fuel: Properties and Principles of the Technology of uranium, Thorium and Plutonium. Pergamon Press, Oxford. [ Links ]
Paper received Aug. 2015
Revised paper received Aug. 2015.














