Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 no.8 Johannesburg Ago. 2015
http://dx.doi.org/10.17159/2411-9717/2015/V115N8A14
Measuring and modelling of density for selected CaO-Al2O3-MgO slags
J.F. XuI; K. WanI; J.Y. ZhangII; Y. ChenI; M.Q. ShengI
IShagang School of Iron and Steel, Soochow University, China
IIShanghai Key Lab. of Modern Metallurgy and Material Processing, Shanghai University, China
SYNOPSIS
The densities of selected CaO-Al2O3-MgO slag systems were measured at 1823K by the Archimedean method. Thirteen different slag compositions were chosen based on different levels of the MgO content and the mass ratio of CaO/Al2O3. The results indicated that the density of the slag decreases with increasing MgO content (from 0-3.78 mass%), but increases with further increases in MgO content up to 11.33 mass%. At a fixed MgO content of 5.5%, the trend of density change with CaO/Al2O3 in the slag is similar to that for changes in MgO content. On the basis of the regular solution approximation rules of excess molar quantities, an attempt was made to estimate the molar volume of the slags investigated. The application of the molar volume model confirmed that the present expanded approximation rules are applicable to predict the molar volumes of the melts discussed.
Keywords: slag density, CaO-Al2O3-MgO system, molar volume, modelling
Introduction
Density is one of the most important fundamental properties of slags, which influences metallurgicaly phenomena in many ways. Density is sensitive to the nature of the chemical bonds and the species in the melt, and is of great importance not only for theoretical research on the structural properties of molten slags, but also for industrial applications. Density is also required to estimate other key properties used to assess the behaviour of high-temperature molten oxides, e.g. viscosity, surface tension, and thermal conductivity.
Liquid calcium aluminate slags that contain magnesia are the basis of most ladle slags for secondary refining processes. The CaO-Al2O3 binary phase diagram (Hallstedt , 1990) shows the eutectic composition (50 wt% CaO and wt% Al2O3) has the lowest melting temperature. The main emphasis of this study is on the 12CaO-7Al2O3 refining slag with MgO additions. The CaO-Al2O3-MgO ternary system is of considerable importance in industrial metallurgical processes, and is fundamental to the understanding of metallurgical slags, ceramic materials, and geological phenomena. Many researchers have reported on the optimization of the ternary CaO-Al2O3-MgO system (Hallstedt, 1995; Jung, Degterov, and Pelton, 2004). It has been shown that the liquidus temperature of the slag is lower than 1823K when the addition of MgO to 12CaO-7Al2O3 is less than 10 wt%. The density of the CaO-Al2O3-MgO system has not been systematically investigated experimentally, and few experimental studies of density have been reported in the CaO-Al2O3-MgO system (Slag Atlas, 1995). Due to the difficulty of experimental measurements at high temperature, the existing data covers only a limited composition and temperature range. In our previous work, the viscosity of the selected CaO-Al2O3-MgO slag system was measured by using the rotating cylinder method and the effects of temperature, the MgO content, and the mass ratio of CaO/Al2O3 were studied. The results indicated that both the MgO content and the mass ratio of CaO/Al2O3 influence the viscosity of the slag (Xu et al., 2011). The aim of the present work is to investigate the effect of MgO additions on the density of 12CaO-7Al2O3 slag. The effects of the MgO content and the mass ratio of CaO/Al2O3 were studied. On the basis of the regular solution approximation rules of excess molar quantities, an attempt was made to estimate the molar volumes of selected CaO-Al2O3-MgO slags investigated.
Experimental
Sample preparation and characterization
The CaO-Al2O3-MgO equilibrium phase diagram from Verlag Stahleisen (Slag Atlas, 1995) is shown in Figure 1. Table I shows the nominal chemical compositions of samples used in the present work. The samples were divided into two groups. In the first group, comprising five samples, the mass ratios of CaO/Al2O3 were equal to unity, while the MgO content was varied from zero to 12 wt%. The eight samples in the second group had MgO contents of 5.5 wt%, and the mass ratio of CaO/Al2O3 was varied from 0.60 to 1.20.
Samples were prepared from CP (chemically pure) grade CaO (>99.0 wt%), Al2O3 (>99.5 wt%), and MgO (>99.0 wt%) powders, which were dried at 373K for 24 hours. The method for preparation of the slag samples has been reported in detail elsewhere (Xu et al., 2012). The powders were ground and weighed to the desired compositions and mixed in a mortar, and the mixtures were melted in a graphite crucible in an air induction furnace for 30 minutes at 1773K. The fused slag samples were poured onto the surface of a cold steel plate. For further homogenization, these samples were then crushed and ground to fine powders. The powder samples were placed in a corundum crucible and were dried and decarburized at 1223K for 30 hours in a muffle furnace in air. Finally, the chemical compositions of the samples were analysed; the results are reported in Table I.
Density measurements
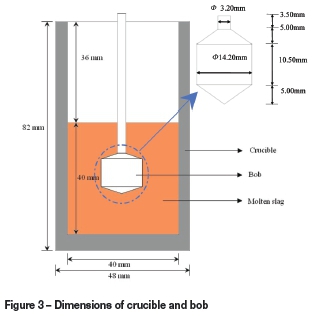
The densities were measured at 1823K by the Archimedean method using the RTW-08 type testing instrument . The experimental set-up and procedure have been described in detail in an earlier publication (Xu et al., 2012). In this section, a brief description of the method is given. The furnace (Figure 2) had a maximum temperature of 1873K. The temperature was measured by a Pt-30Rh/Pt-6Rh thermocouple touching the crucible bottom from outside. The metal bob for measurement was made of molybdenum, which has a melting point of 2896K. The volume of the bob at high temperature was calculated from the values measured in pure water in the temperature range 283-308K and the coefficient of thermal expansion of molybdenum. Dimensions of the crucible and bob are presented in Figure 3. The size of bob had to be carefully designed so that it was applicable in the required measurement range. Purified argon gas (0.2 L/min) was introduced into the reaction chamber during the entire process. The graphite crucible was filled with 120 g slag and placed in the furnace, and the furnace was programmed to heat up to 1823K at a heating rate of 10K/min. The furnace was kept at the target temperature at least for 30 minutes. The densities of the slags were then measured at the predetermined temperature, and the measurements were repeated several times until the values were stable, at which the error was about ±1x10-3 g. After measurement was complete, the furnace was allowed to cool.
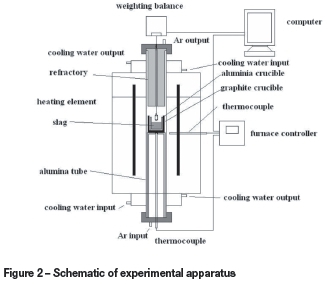
In the density measurements several sources of error may occur. As mentioned above, a deviation in the volume of the bob caused an error of ±0.5% in density, the determination of the temperature with an accuracy of ±5K introduced an error of ±0.3% in density, and the accuracy of ±1x10-3 g in the weighting balance caused an additional error of ±0.3% in density. Since the effect of surface tension on the density measurement is difficult to estimate, no corrections were made for the effect of surface tension of the melt acting on the thin section of the spindle. This has been calculated to cause an error of about 2.0% in the density measurement (Nakanishi et al., 1998). The total error in the determination of the density was less than ±3.1%.
Results and discussion
The density of the selected CaO-Al2O3-MgO slag system was measured at 1823K. Thirteen different slag compositions were chosen based on different levels of MgO content and mass ratios of CaO/Al2O3. The MgO content was varied from 0.39 to 11.33 wt.%, and CaO/Al2O3 mass ratios varied between 0.60 and 1.28. The effects of MgO content and CaO/Al2O3 ratio on the density are shown Figure 4 and Figure 5 respectively.
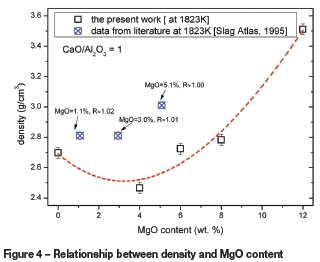
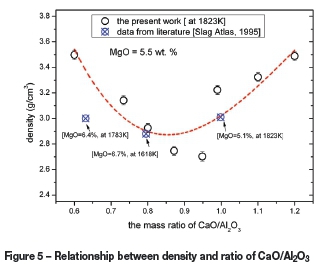
The data for corresponding slags from the literature (Slag Atlas, 1995) is also shown in Figures 4 and Figure 5. Comparison reveals that although the values obtained in the present study may be higher or lower than those from the literature, the density trends are similar. The differences in densities could be related to slag composition and experimental design and procedure. It should be noted that there are difficulties in retrieving reliable experimental density values.
Effect of MgO content on density
The measured densities at a constant mass ratio of CaO/Al2O3 and various amounts of MgO (0.39-11.33%) at 1823K are shown in Figure 4. The results indicate that the density of the slag decreased for low additions of MgO, with a minimum value of 2.47g/cm3 at 1823K at an MgO content of 3.78%. This was followed by a sharp increase in density with further additions of MgO.
An additive method for the estimation of density in alloys and slags has been widely used for some time. The densities of the pure components CaO, MgO, and Al2O3 are 3.32, 3.50, and 3.97g/cm3 respectively (Lide, 2003). Since the density of MgO is much lower than that of Al2O3, slag density will decrease with small increases in MgO content. On the other hand, the addition of metal oxides has a strong impact on the physical properties of molten slag systems. The effect of cations on the structure is commonly related to the charge of the cations as well as the radius. In order to estimate the effect of different cations, the ratios z/r or z/r2are used, where z is the valence and r is the cation radius. Slags are composed of cations and complex anions, and the forces of attraction between ions directly affect slag density (Cui et al., 1996). When MgO is added to 12CaO-7Al2O3-type slags, the complex polymers of aluminum such as AlO45-tetrahedra break down into smaller units, decreasing the degree of polymerization, and the radius of the ions decreases; then the force of attraction between ions and the density of the slag increase (Cui et al., 1996; Mills, 1993). Furthermore, the molar masses of CaO, MgO, and Al2O3 are 56.08, 40.31, and 101.96 g/mol, respectively. Since the molar mass of MgO is lower than that of the other metal oxide components of the slags, when MgO replaces part of the CaO and Al2O3 content in 12CaO-7Al2O3, the total number of ions in the molten slag will increase. This also increases the forces of attraction between the ions, and thus increases the density of the slag.
Effect of CaO/Al2O3 on density
The effect of the mass ratio of CaO/Al2O3 (in the range 0.6-1.28) on density at different temperatures and a constant MgO content of 5.5 wt.% is shown in Figure 5. The effect of CaO/Al2O3 mass ratio on density was the same as the effect of MgO content; density decreases at first, and then increases with increasing the mass ratio of CaO/Al2O3. The minimum density at 1823K was 2.70g/cm3 at a CaO/Al2O3 mass ratio of 0.98.
Since the density of pure CaO is lower than that of Al2O3, increasing CaO/Al2O3 decreases the slag density in line with the additive method of density calculation. On the other hand, with increasing mass ratio of CaO/Al2O3, the network-breaking cations (Ca) present in slag increase, and the complex polymers of aluminum such as AlO45-tetrahedra break down into smaller units, reducing the degree of polymerization, and the radius of ions decreases, thus increasing the attractive forces between ions. Furthermore, the total number of ions in the molten slag will also increase with increasing of the mass ratio of CaO/Al2O3. The lower degree of polymerization and the higher number of ions will enhance the attractive forces between ions, and the density of the slag will increase (Lide, 2003). Due to these effects, the density of slag at first decreases with increasing the mass ratio of CaO/Al2O3, followed by an increase in density with further increases in the mass ratio of CaO/Al2O3. The lower density values of slag at CaO/Al2O3 mass ratios in the range 0.9-1.0 could also be due to the fact that the slag compositions are between the two eutectic points in the CaO-Al2O3-MgO slag system, as shown in Figure 1.
Estimated molar volume of the selected slags
The molar volume, which the reciprocal of the density multiplied by the molar mass, is an important thermodynamic property. Due to the inherent difficulties associated with measurements at high temperature, it is necessary to have access to reliable models for estimating the molar volume of slags, which are reflective of the structure of the melt. There are many kinds of model cited in the literature (Zhang and Chou, 2010; Persson, Matsushita, and Zhang, 2007; Bottinga, Weill, and Richet, 1982; Mills, Yuan, and Jones, 2011; Hayashi, Abas, and Seetharaman, 2004; Priven, 2004; Nakajima, 1994; Vadasz, Havlik, and Danek, 2006; Shu, 2007; Zhang and Chou, 2009) for estimating the molar volume of slag, including physical models and semi-empirical models. Physical models, which are based on the structure of atoms and molecules, can give a clear physical picture of the practical solution. The semi-empirical models combine both theoretical considerations and practical thermodynamics; these models can give more reasonable data and be suitable for many systems with larger compositional ranges.
In order to estimate molar volumes for multi-component silicate melts, expanded approximation rules are proposed, on the basis of the regular solution approximation rules of excess molar quantities for a binary system melt (Vadasz, Havlik, and Danek, 2006). A brief description of the model is given below. Detailed discussions about this method can be found in Vadasz, Havlik, and Danek, (2006).
The molar volume of slags is calculated from Equation [1]:

where Vmis the molar volume of the slag and V{ is the molar volume of the pure componet i, xiis the mole fraction of component i, and VEis the excess molar volume of the slag.
The molar volume of slags can be expressed by the following equation:

Here, xiis the mole fraction of component i, Miis the molecular weight of component i, and ρ is the measured density of the slag.
We have to obtain the relation between the excess molar volume and composition. The regular solution approximation rule is most widely used (Shu, 2007). The excess molar volume can be expressed as follows:
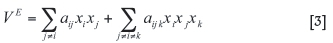
where aij represents the parameters for components i and j, which can be obtained by optimizing an appropriate amount of experimental data in a certain compositional and temperature range. xiand Xj indicate the mole fractions of component i and j, respectively.
The average error of all calculated values can be assessed by using Equations [4] and [5]. δηis the percentage difference between the calculated and measured values. Δ(%)is calculated by taking the summation of all absolute values of δηand dividing by the total number of data.
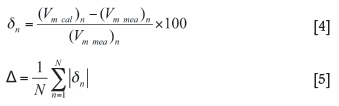
The molar volumes of the pure oxides (Table II) recommended by Mills et al. were used in the present model. Now that all necessary data has been collected, using the experimental data in the CaO-MgO-Al2O3 slag system and its subsystem, the optimized parameters are shown in Table III.

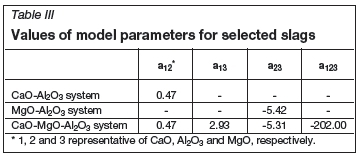
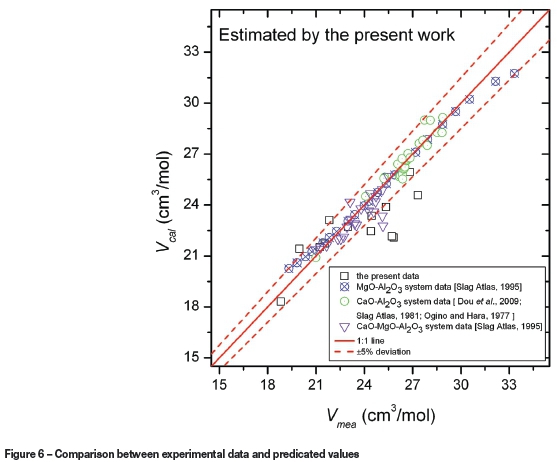
The estimated values using the present model were compared with the experimental data and the data for the CaO-Al2O3 system (Dou et al., 2009; Slag Atlas, 1981; Ogino and Hara, 1977), MgO-Al2O3 system, and the CaO-MgO-Al2O3 system (Slag Atlas, 1995) from the literature to verify the model. The comparison of the experimental data with the model calculated molar volumes are shown in Figure 6. It can be seen that the estimated values agree with the experimental data, and for all calculated values the average error Δ(%) is 2.20%. From the data for the CaO-MgO-Al2O3 slag system and its subsystem, the application of molar volume model confirmed that the present expanded approximation rules are applicable to predict the molar volume of the melts discussed.
Conclusions
Both of the MgO content and the mass ratio of CaO/Al2O3 had an influence on the density of the selected slag. With a mass ratio of CaO/Al2O3 of unity, the density at first decreased with increasing the MgO content, following by an increase.
Changes in the mass ratio of CaO/Al2O3, at a constant MgO content of 5.5 wt.%, gave rise to a similar density trend as changes in the MgO content.
An attempt has been made to estimate the molar volume of the CaO-Al2O3-MgO slag investigated in the present work. Application of the molar volume model confirmed that the expanded approximation rules are applicable for predicting the molar volume of the melts discussed.
Acknowledgements
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (50874072, 51204115), the Natural Science Foundation of Jiangsu Province of China (BK20130308), and the China Postdoctoral Science Foundation (2014M561710).
References
Bottinga, Y., Weill, D.E., and Richet, P. 1982. Density calculations for silicate liquids. I. Revised method for aluminosilicate compositions. Geochimica Cosmochimica Acta, vol. 46, no. 6. pp. 909-919. [ Links ]
Cui, C.M., Xu, X.G., Zhang, X.P., Wang, K.H., and Han ,W.R. 1996, Effect of composition of B2O3-MgO-SiO2-Al2O3 -CaO slag system on physical properties of melt. ActaMetallurgica Sinica, vol. 32, no. 6. pp. 637-641. [ Links ]
Dou, Z.H., Zhang, T.A., Yao, J.M., Jiang, X.L., Niu, L.P., and He, J.C. 2009. Research on properties of Al2O3-CaO slag. Chinese Journal of Process Engineering, vol. 9, no. S1. pp. 246-249. [ Links ]
Hallstedt, Β. 1990. Assessment of the CaO-Al2O3 system. Journal of the American Ceramic Society, vol. 73, no. 1. pp. 15-23. [ Links ]
Hallstedt, Β. 1995, Thermodynamic assessment of the CaO-MgO-Al2O3 system. Journal of the American Ceramic Society, vol. 78, no. 1. pp. 193-198. [ Links ]
Hayashi, M., Abas, R., and Seetharaman, S. 2004. Effect of crystallinity on the thermal diffusivity of mould fluxes for the continuous casting of steels. ISIJ International, vol. 44, no. 4. pp. 691-697. [ Links ]
Jung, I.H., Degterov, S.A., and Pelton, A.D., 2004, Thermodynamic evaluation and optimization of the MgO-Al2O3, CaO-MgO-Al2O3 and MgO-Al2O3-SiO2 systems. Journal of Phase Equilibria and Diffusion, vol. 25, no. 4. pp. 329-345. [ Links ]
Lide, D.R. 2003. CRC handbook of Chemistry and Physics. 84th edn. CRC Press, Boca Raton, Florida. pp. 4-39. [ Links ]
Mills, K.C. 1993. The influence of structure on the physico-chemical properties of slags. ISIJ International, vol. 33, no. 1. pp. 148-155. [ Links ]
Mills, K.C., Yuan, L., and Jones, R.T., 2011, Estimating the physical properties of slags. Journal of the Southern African Institute of Mining and Metallurgy, vol. 111, no. 10. pp. 649-658. [ Links ]
Nakajima, K. 1994. Estimation of molar volume for multicomponent silicate melts. Tetsu-to-Hagane, vol. 80, no. 8. pp. 593-598. [ Links ]
Nakanishi, H., Nakazato, K., Asaba, S., Abe, K., and Maeda, S. 1998. Temperature dependence of density of molten germanium measured by a newly developed Archimedian technique. Journal of Crystal Growth, vol. 191, no. 4. pp. 711-717. [ Links ]
Ogino, K. and Hara, S. 1977. Density, surface tension and electrical conductivity of calcium fluoride based fluxes for electroslag remelting. Tetsu-to-Hagane, vol. 63, no. 13. pp. 2141-2151. [ Links ]
Persson, M., Matsushita, T., and Zhang, J. 2007. Estimation of molar volumes of some binary slags from enthalpies of mixing. Steel Research International, vol. 78, no. 2. pp. 102-108. [ Links ]
Priven, A.I. 2004. General method for calculating the properties of oxide glasses and glass forming melts from their composition and temperature. Glass Technology, vol. 45, no. 6. pp. 244-254. [ Links ]
Shu, Q.F. 2007. A density estimation model for molten silicate slags. High Temperature Material Processes, vol. 26, no. 5-6. pp. 341-347. [ Links ]
Slag Altas. 1981. Verlag Stahleisen, Düsseldorf. p. 224 [ Links ]
Slag Atlas. 1995. 2nd edn.. Verlag Stahleisen, Düsseldorf. pp. 328, 318. [ Links ]
Vadasz, P., Havlik, M., and Danek, V., 2006. Density and surface tension of the systems CaO-FeO-Fe2O3-MgO, CaO-FeO-Fe2O3-ZnO and CaO-Fe2O3-Cu2O. Central European Journal of Chemistry, vol. 4, no. 1. pp. 174-193. [ Links ]
Xu, J.F., Zhang, J.Y., Jie, C., Ruan, F., and Chou, K.C. 2011, Experimental measurements and modeling of viscosity in the CaO-Al2O3-MgO slag system. Ironmaking and Steelmaking, vol. 38, no. 5. pp. 329-337. [ Links ]
Xu, J.F., Zhang, J.Y., Jie, C., Tang, L., and Chou, K.C. 2012. Measuring and modeling of density for selected CaO-MgO-Al2O3-SiO2 slag with low silica. Journal of Iron and Steel Research International, vol. 19, no. 7. pp. 26-32. [ Links ]
Zhang, G.H. and Chou, K.C. 2009. Estimating the excess molar volume using the new generation geometric model. Fluid Phase Equilibria, vol. 286. pp. 28-32. [ Links ]
Zhang, G.H. and Chou, K.C. 2010. Model for evaluating density of molten slag with optical basicity. Journal of Iron and Steel Research International, vol. 17, no. 4. pp. 1-4. [ Links ]
Paper received Oct. 2013
Revised paper received Feb. 2015














