Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.8 Johannesburg Aug. 2015
http://dx.doi.org/10.17159/2411-9717/2015/V115N8A10
Thermogravimetric investigation of macadamia nut shell, coal, and anthracite in different combustion atmospheres
S.O. BadaI; R.M.S. FalconI; L.M. FalconI; M.J. MakhulaII
ISchool of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, South Africa
IIDepartment of Mineral Processing, Mintek, South Africa
SYNOPSIS
The combustion and co-combustion behaviour of macadamia nut shell, high-ash coal, and anthracite, along with their blends was studied using thermogravimetry. The reactivities of all samples were analysed in air, oxygen, and CO2 atmospheres and at different heating rates from 10 to 40°C/min. Macadamia shell was found to have a lower ash content, 0.36%, than coal with 27.49% ash. The calorific values were similar, 19.64 MJ/kg and 19.44 MJ/kg respectively. The differential thermogravimetric results indicate that as the heating rates increase the ignition, peak, and burnout temperatures increase significantly, leading to high combustion rates. The interaction between the fuels was evaluated using the weighted average model, and the results indicated that there is more synergetic interaction between 20% macadamia plus 80% coal under oxygen than in air and CO2 atmospheres. The results of the investigation provide the combustion and co-combustion characteristics of various samples and their blends and indicate their combustion compatibilities.
Keywords: anthracite, blending, coal, macadamia shell, combustion
Introduction
The global macadamia nut industry is developing at a very rapid rate. South Africa is currently the world's third largest producer, with a production of about 35 000 t as of 2012 (Department of Agriculture, Forestry and Fisheries, 2013), compared to 840 t produced in 1996. The greatest numbers of the macadamia nut farms in South Africa can be found in Mpumalanga, Limpopo, and KwaZulu-Natal provinces, with Mpumalanga as the largest producing area. There are many factors influencing the increased demand for macadamia nuts, the most important factor perhaps being their nutritional value (California Dried Fruit and Nuts, 2011; MPC, 2014) and their suitability as a feedstock for manufacturing cosmetics, bakery products, nut paste, chocolates, sauces, and ice-cream. As a fuel, macadamia shell has a high heating value 'as-fired' of about 20.71 MJ/kg (Vhathvarothai et al., 2013), which could in turn be used in drying the nust, and the shell could also be milled and applied as a fertilizer.
The shell makes up almost 60-70% by weight of the macadamia nut. Considerable amounts of shells are generated, which are considered as a waste by the macadamia farmers who have to pay a disposal fee to dump the shells in a landfill. Vhathvarothai et al. (2013) showed that the heating value of macadamia shells is higher than most of the coals used in South African power plants. As the demand for electricity increases, there is need to investigate the co-firing potential of this shell, which is abundantly available in Mpumalanga, the major coal-producing region of South Africa. The utilization of biomass as a single source of fuel for combustion is known to face many challenges, such as the low melting point of the ash, which causes fouling and slagging, varied combustion characteristics, and high moisture and volatile matter contents (Phanphanic and Mani, 2011; Bada et al., 2014a). However, the aforementioned problems could be reduced through the co-blending of biomass with coal in the correct proportions. Studies have shown that the blending of biomass with coal could lead to a significant reduction in emissions of CO2 (Li et al., 2012), and an increase in the combustion reactivity of coal by decreasing the ignition temperature and reducing burnout time (Moon et al., 2013; Bada et al., 2014b).
Numerous thermochemical conversion technologies such as gasification and combustion have proven to be promising ways of producing energy from both coal and biomass. The use of thermogravimetric analysis (TGA) to investigate the thermal characteristics of macadamia nut shell during combustion may provide an insight into the utilization of this resource for power generation in South Africa. With thousands of boilers in South Africa, the co-firing of macadamia and coal might be an added advantage to those using smaller boilers, by reducing their CO2 emissions and improving their energy output. Furthermore, with millions of tons of high-ash coal in South Africa, the co-firing of macadamia might be an attractive option to enhance the thermochemical performance of this low-grade coal. In addition, the success of this approach is likely to develop the possibility of using the considerable tonnages of coal fines generated in the country in a cleaner and more efficient manner. As there is little published literature on thermogravimetric investigations of macadamia combustion, this paper seeks to investigate the viability of using macadamia nut shells as a renewable energy source for combustion and co-combustion applications, and to determine the physiochemical and combustion properties of the material and its co-firing potential with high-ash coal.
In this study, the reactivities of macadamia nut shell, high-ash coal, and anthracite, individually and in blended proportions, were analysed thermogravimetrically in air, oxygen, and CO2 atmospheres at different heating rates. The macadamia/coal and macadamia/anthracite blends were evaluated at different ratios using differential thermogravi-metric (DTG) techniques.
Experimental
Sample preparation
The macadamia material and high-ash coals were sourced from the KwaZulu-Natal province of South Africa. The shell was milled using a Retsch SM 200 cutting mill to -212 pm. The anthracite and the coal samples were milled in a hammer mill and then pulverized to -212 pm, with the representative fractions prepared for the combustion and co-combustion investigation. Blends of macadamia/coal and macadamia/anthracite were prepared using 20%, 50%, and 80% macadamia shell by weight. The calorific values and proximate and ultimate analyses of the individual samples were determined and are presented in Table I.
Thermogravimetric analysis
The combustion and co-combustion of the macadamia nut shell, coal, anthracite and their blends in different weight ratios, were investigated using a TGA 701 Leco thermogravi-metric analyser. The three raw samples were combusted at heating rates of 10, 20, 30 and 40°C/min in an oxygen atmosphere from a temperature of 25°C to 950°C. Thermal combustion and co-combustion of all raw samples and their blends were conducted at a constant heating rate of 10°C/min from 25°C to 950°C under three different atmospheres (air, CO2, and O2) and the samples were held at 950°C until no further weight loss was recorded. For each experiment, 80 mg of fuel was used. The derivative thermogravimetric (DTG) curve generated provided information on the reactivities of the individual samples and blends under these different conditions.
Material characteristics and analysis of sample interaction
The proximate analyses for all samples were conducted in accordance with ASTM D-5142, with approximately 1 g of material used in determining the inherent moisture, ash content, and volatile matter present, and fixed carbon calculated by difference. The calorific value was determined for all samples using a Leco AC500 calorimeter in accordance with ASTM D5865-04. The system uses an electronic thermometer with an accuracy of 0.0001°C to measure the temperature every six seconds, with the results obtained within 4.5 to 7.5 minutes. Ultimate and sulphur analyses of all samples were performed according to ASTM D 5373-02 and ASTM D 4239-05 for CHN and sulphur content respectively, using a Leco CHN 628 with add-on 628 S module.
The experimental DTG profiles obtained from the co-combustion of the fuels at different weight proportions were used to study the interaction between coal and macadamia nut shell. The theoretical DTG curves of the various blends were calculated by summing the weight loss rates of each individual sample and comparison with the results from the experimental DTG curves. This sought to confirm whether synergistic interactions occurred between the macadamia and coal components in the blends. The theoretical relationship used was defined as follows (Gil et al., 2010; Wang et al.,2011):
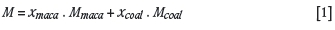
where xmacaand xcoai are the fractions of macadamia and coal in the blend, respectively, and Mmacaand Mcoai are the weight loss rates (%/min) of the individual fuels in the blend.
Results and discussion
Characteristics of Coal A, raw macadamia, and thermally treated PA
The results of the proximate and ultimate analyses and the calorific values of the macadamia nut shell, coal, and anthracite are depicted in Table I. The ash content of the macadamia shell was found to be 0.36%, compared to the high ash content of coal (27.49%). The ash content of the shell was found to be much lower than that of most woody and non-woody biomass feedstocks (Xiang etal., 2012; Idris et al., 2012; Slopiecka et al., 2012; Bada et al., 2014a, 2015).
The fixed carbon content of the anthracite sample was significantly higher than that of the coal and the macadamia nut shell. The volatile matter and moisture contents of the macadamia were higher than the values for coal. Both samples had similar calorific values. The nitrogen and sulphur contents of the macadamia nut shell were very low at 0.30%, compared with 1.36 % nitrogen and 0.76 %, sulphur in the coal. The lower nitrogen content of the macadamia shell could support its application as a fuel in combustion systems, thereby reducing the emission of NOx in the flue gas when co-fired with coal.
Influence of different heating rates on combustion curves in an O2 atmosphere
The DTG curves at different heating rates (10 to 40°C/min) in oxygen for raw macadamia, coal, and anthracite are presented in Figure 1. The characteristic parameters of the fuels, i.e. ignition, peak, and burnout temperature, are shown in Table II. The DTG profile presented two peaks for the raw macadamia at a heating rate of 10 to 40°C/min. The intensity of the shoulders was more pronounced for the macadamia sample at 10°C/min compared to other heating rates (Figure 1a). The shoulder observed in the temperature region of 230-340°C for the curve at 10°C/min could be attributed to the decomposition of the low molecular weight compound 'hemicellulose and some cellulose', with the evolution of gases such as CO and CO2. The minor peak seen in the region of 380-460°C for the curve at 10°C/min can be ascribed to the complete degradation of the organic functional groups of the macadamia nut shell. This observation is in agreement with the literature (Yang et al., 2007; Pickard et al., 2014).
A decrease in the intensity of the second peak was observed as the heating rate increases from 10 to 40°C/min. This could be a result of the decrease in time for devolatilization to occur and a reduction in reaction residence time. It was also observed that as the heating rate increases, the DTG curves for all samples move into a higher temperature region, with a wider combustion range at higher temperatures.
This is considered to be due to the limitation of heat transfer to the inner part of the sample for effective combustion at the higher heating rates. With lower heating rates, more effective heat would be transferred steadily into the inner part of the sample, resulting in more rapid decomposition of the sample (Xie and Ma, 2013). Based on the thermal characteristic data (Table II), it can be seen that as the heating rate increases, the ignition, peak, and burnout temperatures increase significantly in the case of all the samples (macadamia, coal, and anthracite). In addition, an increase in reactivity (%/min) was noted as the heating rate increases, which indicates that combustion intensity is enhanced by higher heating rates (Chen et al., 2012; Xie and Ma, 2013).
Influence of different atmospheres on combustion at 10°C/min
The TG and DTG curves for raw macadamia, coal, and anthracite at a heating rate of 10°C/min under three different atmospheres are presented in Figures 2 and 3. All samples followed the same trend of moisture removal. As the temperature increases, ignition of the hemicellulose content of the macadamia occurs at a temperature range of around 160 to 164°C under all three atmospheres. The peaks are seen overlaying each other, with the sample under an oxygen atmosphere igniting first and having the highest reactivity of 9.11%/min (Table II). The second peaks observed for the three samples (Figure 3) show distinctive curves under different atmospheres. This could be attributed to the differences in the chemical composition and thermal characteristics of the organic functional group within the samples and their individual interaction with the gases used. A significant mass loss of 56.30% was obtained for macadamia combusted under an oxygen atmosphere, followed by 44.31%, in CO2 and 47.86% in air . The same trend was observed for coal and anthracite. The combustion of all samples in oxygen occured in a lower temperature region with the highest reactivity and shortest burnout time, which is in line with the findings of Pickard etal. (2014). In regard to the burning profile of anthracite in oxygen, the peak temperature was attained at 502.85°C, while in air and CO2 the peaks were attained at temperatures of 612.52°C and 654.53°C, respectively. Figure 2 also shows the TG profiles for the three samples in 100% CO2 atmosphere. The weight loss profiles for the coal and anthracite in CO2 atmospheres, as well as with their DTG curves (Figure 3), were similar, and different from that of macadamia in a CO2 atmosphere. The thermal degradation of macadamia is completed over a wider temperature range compared to coal and anthracite (Figure 3). In summary, significant differences in fuel mass loss rate, ignition time, and burnout time were seen to be due to the influence of the different combustion atmospheres on each product tested. The differences between the fuel combustibility at ignition and burnout are likely to be a result of compositional differences, such as volatile matter and fixed carbon content, between the samples.


Co-combustion of different fuel blends at 10°C/min under air, CO2 and O2 atmospheres
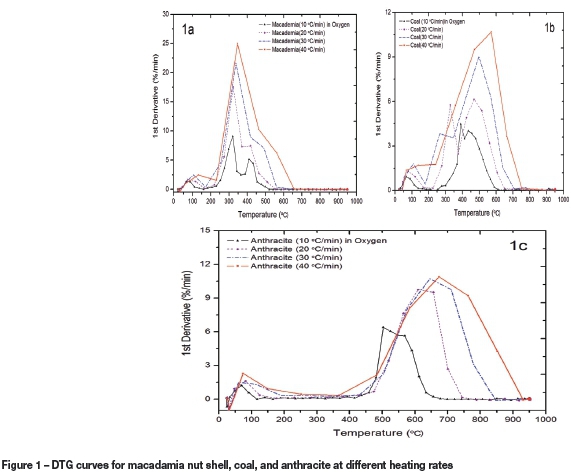
The co-combustion of coal and anthracite with 20, 50, and 80 wt.% macadamia was investigated under three different atmospheres. The thermal profiles obtained from the TGA tests were used to compare the combustibilities of the fuels as shown in Figures 4-6. The thermographs illustrate the high combustibility of the macadamia/coal and macadamia/anthracite blends. Figure 4 shows that the co- combustion of 20% macadamia in the coal blend has the highest reactivity of about 12.50 %/min in oxygen compared to the tests under CO2 and air. The 50% and 80% coal samples in the macadamia blends have a higher reactivity in the lower temperature region than the raw macadamia and raw coal samples. Furthermore, Figure 4 shows that 50% and 20% anthracite blended with raw macadamia combusts in the same temperature range as the raw macadamia. Blending with macadamia shell resulted in a significant improvement in the combustibility of both coal and anthracite, as seen by the decrease in their ignition temperatures. The ignition temperature was decreased from 415°C (raw anthracite) to around 220°C in co-firing with macadamia nut shell.

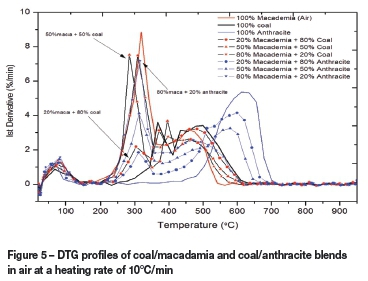

The DTG profiles presented in Figures 5 and 6 illustrate the thermographs of coal/macadamia and coal/anthracite blends in air and CO2 respectively. In air, raw macadamia nut shell had the highest reactivity of 8.85 %/min and the lowest burnout temperature. This is in contrast to results obtained under oxygen, where 20% macadamia plus 80% coal had the highest reactivity. It was observed that all coal/macadamia blended samples have lower burnout and ignition temperatures in the lower temperature zones compared to coal. In addition, the anthracite/macadamia blends also followed the same trend under the air atmosphere, with 80% macadamia plus 20% anthracite having a higher reactivity and lower burnout temperature than the raw coal. Under the CO2 atmosphere (Figure 6), the sample with the lowest coal ratio in macadamia/coal blends (80% macadamia plus 20% coal) was seen to have a similar mass loss rate to that of the raw macadamia. In summary, blending with macadamia shells resulted in a significant improvement in the combustion of the high-ash coal and anthracite samples. The second 'shoulder' peaks seen for all samples tested decreased as the percentage of coal and anthracite in the blends decreased.
Interaction between the blends of macadamia and coal under air, 002 and O2
The calculated and experimental DTG curves were compared with the aim of determining the combustion behaviour of macadamia shell and coal when blended at different ratios, and under different atmospheres. The calculated DTG curves were derived from Equation [1] as the weighted average of the individual fuels in the decomposition. The interaction between the blends of macadamia and coal at 10°C/min, i.e. the experimental total weight loss rate (%/min), could be assumed to be additive or non-synergistic if it is equal to the calculated weighted average derived from Equation [1]. The calculated and experimental DTG curves for 20% macadamia and 50% macadamia blends with coal at a heating rate of 10°C/min are depicted in Figure 7. The calculated and experimental DTG curves for 20% macadamia plus 80% coal under all atmospheres show good agreement at the initial and burnout stages. Significant deviation of about 12%/min was noted for the calculated and experimental 20% macadamia plus 80% coal sample under oxygen during the main mass-loss stage at a temperature above 240°C. The experimental weight loss rate (%/min) lagged behind the calculated weight loss rate, and the DTG curve was shifted to a lower temperature. The deviations observed for samples under air and CO2 atmospheres were not as significant as that under oxygen, indicating there is more synergetic interaction between macadamia and coal under an oxygen atmosphere.

As the percentage of macadamia shell in the blend increases to from 20% to 50%, as shown in Figure 8, the deviation in the experimental and calculated DTG curves of the samples in air and CO2 atmosphere increases (compare with Figure 7). The ignition, peak, and burnout temperatures for the experimental DTG curves for the three samples are shifted to lower temperatures. From a qualitative point of view, the experimental curves exhibit significant deviations from the theoretical curves, particularly in the temperature range of 240-630 °C. This indicates that the interaction between macadamia and coal is more pronounced at higher temperatures. It is suggested that the deviation between the calculated and experimental DTG curves at higher temperature could be a result of the gases and heat released during the decomposition of the macadamia, which accelerated the combustion of coal in the blend.

Conclusion
The results suggest that macadamia shells may constitute a valuable and efficient source of biomass for co-firing with coal and anthracite. The thermographs obtained from the co-combustion of macadamia/coal blends at 20%, 50% and 80% weight percentage macadamia in oxygen showed that all the blends have higher reactivities than coal alone, and that the high reactivities occur in the lower temperature regions. Combustion in air is slightly less efficient, but may be more economical than in oxygen. Anthracite combusts in a much higher temperature range than coal and macadamia, and therefore may constitute a long-term compatible blend component.
Acknowledgements
The authors gratefully acknowledge the financial support of the NRF SARChI Clean Coal Technology and the University of the Witwatersrand. We thank the Mineral Processing Department, Mintek for permission to publish and for access to some of their research facilities.
References
Bada, S.O., Falcon, R.M.S., and Falcon, L.M. 2014a. Investigation of combustion and co-combustion characteristics of raw and thermal treated bamboo with thermal gravimetric analysis. Thermochimica Acta, vol. 589. pp. 207-214. [ Links ]
Bada, S.O., Falcon, R.M.S., Falcon, L.M., and Bergmann, C.P. 2014b. Co-firing potential of raw and thermally-treated Phyllostachys aurea bamboo with coal. Energy Sources, Part A. (In press). [ Links ]
Bada, S.O., Falcon, R.M.S., and Falcon, L.M. 2015. Characterization and co- firing potential of a high ash coal with Bambusa balcooa. Fuel, vol. 151. pp. 130-138. [ Links ]
California Dried Fruit and Nuts. 2011. Nut Crop Report, 2011. http://www.stapleton-spence.com/crop-reports/june-2011-nut-crop-report/ [Accessed 8 September 2014]. [ Links ]
Chen, C.X., Ma, X.Q., and He, Y. 2012. Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA. Bioresource Technology, vol. 117. pp. 264-273. [ Links ]
Department of Agriculture, Forestry and Fisheries, South Africa. A profile of the South African macadamia nuts market value chain, 2013. Pretoria. [ Links ]
Gil, M., Casal, D., Pevida, C., Pis, J., and Rubiera, F. 2010. Thermal behaviour and kinetics of coal/biomass blends during co-combustion. Bioresource Technology, vol. 101, no. 14. pp. 5601-5608. [ Links ]
Idris, S.S., Rahman, N.A, and Ismail, K. 2012. Combustion characteristics of Malaysian oil palm biomass, sub-bituminous coal and their respective blends via thermogravimetric analysis (TGA). Bioresource Technology, vol. 123. pp. 581-591. [ Links ]
Lí, J., Brzdekiewicz, A., Yang, W., and Blasiak, W. 2012. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Applied Energy, vol. 99. pp. 344-354. [ Links ]
Macademia Processing Companies (MPC) Ltd. 2014. Macademia kernel in various market segments. http://www.mpcmacs.com.au/wholesaleproducts.html [Accessed 28 August 2014]. [ Links ]
Moon, C., Sung, Y., Ahn, S., Kim, T., Choi, G., and Kim, D. 2014. Effect of blending ratio on combustion performance in blends of biomass and coals of different ranks. Experimental Thermal and Fluid Science, vol. 47. pp. 232-240 [ Links ]
Phanphanich, M. and Mani, S. 2011. Impact of torrefaction on the grindability and fuel characteristics of forest biomass. Bioresource Technology, vol. 102, no. 2. pp. 1246-1253. [ Links ]
Pickard, S.C., Daood, S.S., Pourkashanian, M., and Nimmo, W. 2014. Co-firing coal with biomass in oxygen- and carbon dioxide-enriched atmospheres for CCS applications. Fuel, vol. 137. pp.185-192. [ Links ]
Slopiecka, K., Bartocci, P., and Fantozzi, F. 2012. Thermogravimetric analysis and kinetic study of polar wood pyrolysis. Applied Energy, vol. 97. pp. 491-497. [ Links ]
Vhathvarothai, N., Ness, J., and Yu, J. 2013. An investigation of thermal behaviour of biomass and coal during co-combustion using thermogravi-metric analysis (TGA). International Journal of Energy Research, vol. 38, no. 6. pp. 804-812. [ Links ]
Wang, Q., Zhao, W., Liu, H., Jia, C., and Lí, S. 2011. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion. Applied Energy, vol. 88, no. 6. pp. 2080-2087. [ Links ]
Xiang, F. and Lí, J., 2012. Experimental study on ash fusion characteristics of biomass. Bioresource Technology, vol. 104. pp. 769-774. [ Links ]
Xie, Z. and Ma, X. 2013. The thermal behaviour of the co-combustion between paper sludge and rice straw. Bioresource Technology, vol. 146. pp. 611-618. [ Links ]
Yang, H., Yan, R., Chen, H., Lee, D.H., and Zheng, C. 2007. Characteristic of hemicellulose, cellulose and lignin pyrolysis. Fuel, vol. 86. pp. 1781-1788. [ Links ]
Paper received Nov. 2014
Revised paper received Feb. 2015














