Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 no.8 Johannesburg Ago. 2015
http://dx.doi.org/10.17159/2411-9717/2015/V115N8A6
Comparison of physical properties of oxidative sintered pellets produced with UG2 or metallurgical-grade South African chromite: A case study
R.I. GlastonburyI; J.P. BeukesI; P.G van ZylI; L.N. SadikitII; A. JordaanI; Q.P. CampbellI; H.M. StewartIII; N.F. DawsonIII
IChemical Resource Beneficiation, North-West University, Potchefstroom
IIMinerals Processing Division, Mintek
IIIGlencore Alloys, South Africa
SYNOPSIS
The physical properties of oxidative sintered pellets produced from typical South African UG2 ore are compared with the physical properties of pellets produced with conventional South African metallurgical-grade chromite ore (from the Lower Group 6 or the Middle Group 1 and 2 seams). A statistical evaluation of the cured (sintered) compressive strengths proved that pellets prepared from UG2 ore are likely to have the same, or better, compressive strength than pellets prepared from metallurgical-grade chromite ore. The cured abrasion strength of the UG2 pellets was also superior to that of the metallurgical-grade pellets. Scanning electron microscopy (SEM) backscatter, secondary electron, and elemental X-ray mapping were used to determine the reasons for the general superior strength of the UG2 pellets. The case study UG2 ore also required 13 kWh/t less energy for milling to attain the required particle size distribution prior to pelletization, which can lead to substantial cost savings. Results presented in this paper can be utilized by ferrochromium (FeCr) producers to better quantify the advantages and disadvantages associated with the use of UG2 ore for FeCr production.
Keywords: South Africa, UG2, metallurgical-grade chromite, oxidative sintered pellets, compressive and abrasion strengths, milling energy requirements
Introduction
Chromite, a mineral of the spinel group, is the only commercially viable source of virgin chromium units. Geologically, commercial chromite deposits in the world are found in three forms, i.e. alluvial-, podiform-, and stratiform-type deposits (Murthy et al., 2011; Cramer et al., 2004). Alluvial deposits were formed by weathering of chromite-bearing rock with the subsequent release of chromite and gravity concentration by flowing water. However, these deposits are relatively small and of comparatively minor commercial interest. Podiform-type chromite deposits usually occur as irregularly shaped pods or lenses, and their distribution within a mineralized zone is usually relatively erratic and unpredictable, making the exploration of these deposits difficult and costly (Cramer et al., 2004). Stratiform-type chromite deposits occur as parallel seams in large, layered igneous rock complexes with more regular layering and lateral continuity (Cramer et al., 2004). The largest example of a stratiform-type chromite deposit is the Bushveld Complex (BC) in South Africa, which holds an estimated three-quarters of the world's viable chromite ore resources (Cramer et al., 2004).
Several chromite seams exist in the BC. The seams of economic interest are the Lower Group 6 (LG6), the Middle Group 1 and 2 (MG1 and 2), and the Upper Group 2 (UG2) seams (Cramer et al., 2004). The LG and MG seams are specifically exploited for their chromium content, while the UG2 seam is mined primarily as a source of platinum group minerals (PGMs) (Xiao and Laplante 2004). Extraction of the PGMs from the UG2 ore usually involves the liberation of the sulphide by milling and subsequent recovery of the PGM concentrate through flotation (Xiao and Laplante, 2004). Chromite in the PGM concentrate is undesirable; therefore, PGM recovery circuits are specifically designed to ensure maximum rejection of the chromite to the tailings stream. However, the chromite in the PGM tailings can potentially serve as feedstock for ferrochrome (FeCr) production, after beneficiation to increase the chromium content.
South Africa has dominated global production of FeCr in the recent past (Kleynhans et al., 2012 and references therein) due to its vast chromite resources and past history of relatively inexpensive electricity. FeCr is a relatively crude alloy, consisting predominantly of iron (Fe) and chromium (Cr), which is utilized mainly for the production of stainless steel. In South Africa, FeCr is generally produced from metallurgical-grade chromite ore originating from the LG or MG seams. However, due to the increase in the availability of UG2 ore, as well as technical innovations in the FeCr and stainless steel industries, an increasing volume of UG2 feed material is being utilized by South African FeCr producers (Cramer et al., 2004). However, there are certain advantages and disadvantages that need to be considered. The price that a FeCr producer is prepared to pay for UG2 is determined by balancing these advantages and possible disadvantages. Advantages related to the use of UG2 by South African FeCr producers include:
> The utilization of UG2 for FeCr production minimizes tailing volumes for PGM producers, which also has associated financial benefits, e.g. smaller tailing storage facilities
> The use of UG2 contributes to the optimum utilization of natural resources, since additional mining is avoided. This also reduces the carbon footprint of FeCr producers
> The overall safety risk associated with FeCr production is much lower if the mining of new chromite units can be reduced by the utilization of upgraded UG2 tailings from PGM operations. Although this is not a direct financial or technical benefit, environmental-, health-and safety-related aspects are as important as production volumes and profit margins in the modern-day industrial setting
> UG2 ore is usually cheaper than conventional metallurgical chromite ore from LG6 or the MG1 and 2 seams.
The disadvantages associated with the substitution of metallurgical-grade chromite ore with UG2 during FeCr production include:
> The Cr/Fe ratio of UG2 ore is lower than that of metallurgical-grade ore from LG6 or the MG1 and 2 seams (Cramer et al., 2004). As well as Fe and Cr, chromite also contains magnesium (Mg) and aluminium (Al) in varying proportions, depending on the deposit. The chemical composition of chromite can be represented by the formula (Mg, Fe2+) (Cr, Al, Fe3+)2O4 (Murthy et al., 2011). In this structure, Fe2+ can be replaced by Mg and similarly Cr can be replaced by Fe3+ and Al. This variation in composition can result in substantial differences in the Cr/Fe ratio of chromite deposits. Specifically within the BC, the compositional variation within a particular seam is relatively small across the whole Complex, but there is a progressive shift in composition between the sequential reef horizons. Since Fe oxides are more easily reduced (at lower temperatures) than Cr oxide (Niemelá et al., 2004), Fe recovery from chromite ore is higher than that of Cr. Therefore, the lower Cr/Fe ratio of UG2 ores results in a lower Cr content of the FeCr produced. Since FeCr producers are paid only for the Cr content of FeCr, UG2 utilization has a disadvantage in this regard
> The above-mentioned dilution of Cr content in the FeCr produced from UG2 also results in higher transport costs per Cr unit. This is significant, considering that most of South Africa's FeCr is exported to stainless steel producers in Europe, Asia, and North America. This additional transport cost also increases the overall carbon footprint of FeCr production in South Africa
> Cramer et al. (2004) reported that the higher Ti content of UG2 ore could also be problematic, since some stainless steel producers have specifications for the Ti content of FeCr.
FeCr is produced in South Africa with various process combinations, which were recently reviewed by Beukes et al. (2010). At least eight of the 14 South African FeCr smelters utilize oxidative sintered pelletized feed. As described by Beukes et al. (op. cit.), this process entails the wet milling of chromite, together with a small percentage of carbonaceous material, followed by de-watering. Refind clay, which serves as a binder is then added and mixed into the moist milled material. The mixture is then pelletized in a pelletizing drum. The over- and undersized green pellets are removed and recycled, while the appropriately-sized green pellets are layered on a sintering belt, which is protected by a layer of previously sintered pellets. The green pellets are then ignited in a furnace, and air is passed through the pellet bed to sinter the pellets. The amount of carbon present in the green pellets is limited to supply just enough exothermic energy to sinter the pellets properly (Beukes et al., 2010).
The oxidative sintered pelletized feed technology is commonly applied in the South African FeCr industry, and beneficiated UG2 ore is increasingly being used as a feedstock into this process. However, a direct comparison of the physical properties of oxidative sintered pellets produced from UG2 with the properties of pellets produced with conventional metallurgical-grade chromite ore (from the LG6 or the MG1 and 2 seams) is currently lacking in the peer-reviewed scientific literature. Singh and Rao (2008) reported the impacts of properties of different chromite ores of Indian origin on pelletization and sintering. Although knowledge can be gained from similar studies, it cannot be used to exactly predict the performance of South African UG2 ore in the oxidative sintered process. This knowledge gap was recently highlighted by a large South African FeCr producer that requested the authors to investigate this matter. This producer indicated that some negative aspects associated with the physical properties of oxidative sintered pellets produced from UG2 ore were being reported by operational personnel. This had resulted in some operational resistance to the use of large proportions of ore from the UG2 horizon in the oxidative sintered process. In this paper, the physical properties of oxidative sintered pellets produced from a typical South African BC metallurgical-grade ore are compared to the properties of oxidative sintered pellets produced from a typical South African UG2 ore.
Experimental
Materials
A UG2 ore sample and a metallurgical-grade ore sample (from the LG6 seam) were received from one of the largest FeCr smelters situated in the western limb of the BC. At the time when this study was initiated, these specific ores were used for the production of oxidative sintered pellets at this smelter. Refined bentonite (G&W Base (Pty) Ltd., 2013), which was used as a binder during the pelletization process associated with the oxidative sinter pellet production, was obtained from the same FeCr producer. Anthracite from Nkomati Anthracite (Pty) Ltd, Mpumalanga Province, South Africa (Sentula, 2013) was used as a carbonaceous source in the pellets.
In addition, 10 milled chromite ore samples were obtained from a large FeCr producer applying the oxidative sintered process. These samples were used only to determine the particle size requirement during milling of chromite ore in preparation for the production of oxidative sintered pellets.
Surface, crystalline, and chemical analyses
Scanning electron microscopy (SEM) using energy-dispersive X-ray spectroscopy (EDS), utilizing an FEI Quanta 200 instrument with an integrated Oxford Instruments INCA 200 EDS microanalysis system, was utilized to perform surface analysis of the case study materials, i.e. visual inspection and surface chemical characterization. Chemical characterization of chromite ore samples was performed with a Spectro Ciros Vision inductively coupled plasma optical emission spectrometer (ICP-OES). Semi-quantitative and quantitative X-ray diffraction (XRD) analyses were conducted with a Philips X-ray diffractometer (PW 3040/60 X'Pert Pro) to determine the mineral composition of the UG2 and metallurgical-grade chromite ores. The samples were scanned using X-rays generated by a copper (Cu) Κα X-ray tube. The measurements were carried out between variable divergence and fixed receiving slits. The crystalline phases were identified using X'Pert Highscore Plus software, while the relative phase amounts were estimated using the Rietveld method (Autoquan programme). X-ray fluorescence (XRF) was also used to determine the concentration of elements present in the case study materials, using the same instrument as for XRD analysis, with a rhodium (Rh) X-ray tube and a Super Q database.
Milling
A Siebtechnik mill was used to reduce the materials (ore, anthracite, and bentonite) to the required particle size prior to pelletization. All parts of this mill that made contact with the charge made of tungsten carbide, which prevented possible Fe contamination of the milled material. Prior to milling, the materials were dried at 40°C for one day and then cooled in airtight containers to avoid possible moisture absorption. The three components were mixed in a ratio of 97 wt% ore, 2 wt% anthracite and 1 wt% bentonite. Batches of 170 g of this dried material mixture were then milled. After a batch had been milled, the milled material mixture was collected in a sample bag and stored for future usage. The mill was cleaned before milling of a new batch commenced.
In order to quantify the energy requirements during the milling of the two different case study ores, stirred mill tests were conducted. These were carried out utilizing a mill with a 5 L stationary vertical chamber and a stirrer consisting of a shaft fitted with pins to agitate the grinding media. The stirrer rotational speed was held constant at 400 r/min (3.7 m/s). The grinding chamber was 18 cm in diameter and 23 cm high. The mill was equipped with a variable speed drive and a torque sensor to facilitate the accurate determination of energy absorbed by the charge. All the key operating conditions were monitored with a computer that also calculated the energy consumption. The grinding chamber was equipped with a water jacket for cooling. 1 kg of ore was milled with 1.5 kg of water and 20 kg of 6 mm steel balls. For each case study ore, five different milling energy inputs were tested experimentally, i.e. 10, 20, 40, 80 and 160 kWh/t. In practice (industrial application), a relatively narrow energy input range would be expected. However, this relatively large range was tested in order to identify possible trends.
Particle size analysis
Particle size distribution of the as-received case study ores was determined by wet screening. The particle size distributions of the milled chromite ore samples generated with the two different milling methods were determined by laser diffraction particle sizing using a Malvern Mastersizer 2000. A diluted suspension of milled material was treated ultrason-ically for 60 seconds prior to the measurement in order to disperse the individual particles and to avoid the use of a chemical dispersant.
Pelletization
The milled material mixtures (obtained from the Siebtechnik mill) were pressed into cylindrical pellets in a Specac PT No. 3000 13 mm die with an LRX Plus strength-testing machine (Ametek Lloyd Instruments) equipped with a 5 kN load cell. Each pellet consisted of 3 g dry milled material to which two drops of water were added prior to pressing the pellet, i.e. a moisture content of approximately 4.1 wt%. The compression rate was controlled at 10 mm/min until a load of 1.5 kN was reached, and this load was then held for 30 seconds. Although time-consuming (each pellet was made individually), this technique was preferred over conventional disc or drum pelletization, which can result in the formation of pellets with different densities and sizes. Pressing the pellets according to the procedure described here ensured consistent density and size, allowing a monovariance investigation of other process parameters.
Oxidative sintering
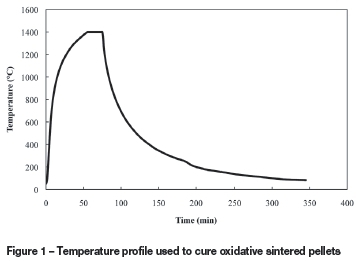
A camber furnace (Lenton Elite, UK, model BRF 15/5) with a programmable temperature controller was used to conduct all oxidative sintering experiments. The temperature profile used in this experimental study was compiled in an attempt to simulate conditions occurring in the industrial oxidative sintering process. Figure 1 depicts the temperature profile that was utilized. The furnace was heated up at the maximum rate to 1 400°C, and this temperature was maintained for 20 minutes. The furnace was then switched off and the pellets allowed to cool inside the furnace. After 100 minutes, the furnace door was opened to accelerate the cooling of the pellets.
Compressive strength testing
The compressive strength of the oxidative sintered pellets was tested with an Ametek Lloyd Instruments LRXplus strength tester. The speed of the compression plates was maintained at 10 mm/min during crushing to apply an increasing force on the pellets. The maximum force applied to incur breakage was recorded for each pellet.
Abrasion resistance testing
The abrasion resistance test apparatus utilized was based on a downscaled version of the European standard EN 15051 rotating drum, similar that used by Kleynhans et al. (2012) and Neizel et al. (2013). The drum was designed according to specifications provided by Schneider and Jensen (2008). Abrasion resistance tests were conducted at drum rotational speeds of 33, 55, and 77 r/min. A batch of ten oxidative sintered pellets, generated under specific experimental conditions, was abraded for different time periods, i.e. 1, 2, 4, 8, 16, 32, and 64 minutes. After each time interval, the material was screened at 9.5 mm. The over- and undersized material was then weighed and all the material returned to the drum for further abrasion until the final abrasion time interval was reached.
Statistical handling of data
The compressive strength tests were repeated 50 times for each of the experimental conditions investigated. In order to determine whether the differences in mean values between the compressive strength of the two case study ores were meaningful, the t-test was applied (Skoog et al., 2004).
Results and discussion
Material characterization
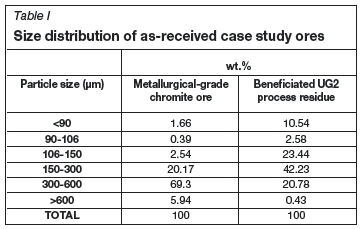
The particle size distributions of the as-received case study UG2 and metallurgical-grade chromite ore samples are presented in Table I. It is evident from these results that the UG2 ore was substantially finer than the metallurgical-grade chromite ore. This was expected, since the UG2 ore originated from the process residue of the PGM industry and had already been milled to liberate the PGMs. The relevance of the difference in size distribution of the as-received case study ores will be discussed later.
In Table II, the chemical, surface chemical, and crystalline compositions of the two case study ores are presented. Chemical analysis (ICP-OES) indicated that the Cr2O3 contents of the metallurgical grade and UG2 case study ores were 44.19 and 41.82%, respectively, which is typical for these ores (Cramer et al., 2004). Slightly lower Cr2O3 contents were obtained for both case study ores with XRF analysis, which can be expected since results obtained from the destructive chemical analysis technique (ICP-OES) will be more accurate. As expected, the XRD analyses indicated that chromite was the dominant Cr-containing mineral phase. All chemical analytical techniques (ICP-OES, SEM-EDS, and XRF) indicated that the Fe content of the UG2 ore was higher than that of the metallurgical-grade chromite ore, which was reflected in the Cr/Fe ratios of 1.4 and 1.58 respectively. These Cr/Fe ratios are quite typical of South African metallurgical-grade and UG2 ores that are used in FeCr production (Cramer et al., 2004).
The bentonite used as a binder and the anthracite used as a carbonaceous source in the oxidative sintered pellets were exactly the same sample materials utilized by Kleynhans et al. (2012). Kleynhans et al. conducted detailed characterization of these two materials (chemical, surface chemical, and crystalline contents) and this data is therefore not presented in this paper. In summary, the bentonite consisted mostly of smectite mineral groups, which were made up of montmorillonite. On an air-dried basis, the anthracite sample contained 75.08% fixed carbon, 17.79% ash, and 6.87% volatiles.
Particle size requirement
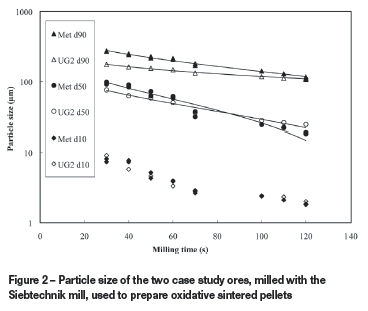
For the pelletized chromite pre-reduction process, Kleynhans et al. (2012) stated that the milled particle size requirement is 90% of the particles (d90) to be smaller than 75 μm. However, definitive information regarding the particle size requirement for oxidative sintered pellets could not be obtained in the peer-reviewed public domain. Laser diffraction particle size analyses of 10 milled chromite ore samples obtained from a large FeCr producer applying the oxidative sintered process yielded an average d90 of 108 μm. This value was therefore adopted as the required particles size target during subsequent milling experiments.
Figure 2 presents the d10, d50, and d90 values obtained by laser diffraction particle size analysis of the two case study ores milled for different time periods with the Siebtechnik mill. The solid lines indicate the trends of each data series. Although the d50 of both the case study ores was the same after approximately 95 seconds of milling, the d90 of the metallurgical-grade chromite ore at this milling time was still higher than the target value of 108 μm. After approximately 120 seconds of milling, both case study ores had a d90 close to the aforementioned target value. However, at this milling time, the d50 values differed substantially. This data indicated that it would be impossible with this specific mill to find a milling time that would yield a d90 value of 108 μm for both ores, with both ores also having the same particle size distribution. This might have been possible if the composition of the milling media could have been changed, but this was impossible with the mill utilized. It was therefore decided to mill the case study ore mixtures for both 95 and 120 seconds in preparation for pelletization.
Compressive strength comparison
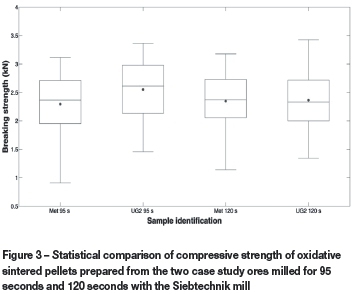
In Figure 3, the compressive strength of oxidative sintered pellets prepared from the two case study ores milled with the Siebtechnik mill for 95 seconds ( i.e. with the same d50 values) and for 120 seconds (i.e. with similar d90 values), is presented. In this box-and-whisker plot, the short horizontal line inside the box indicates the median of each data-set, the dot the mean, the upper and lower ends of the boxes the 25th and 75th percentiles, and the whiskers 2.7σ (99.3% coverage if it is assumed that the data has a normal distribution). Although there are significant overlaps between the datasets, it seems that the oxidative sintered pellets prepared from UG2 ore milled for 95 seconds were slightly stronger ( i.e. higher median and mean values) in relation to the oxidative sintered pellets prepared from the metallurgical-grade chromite ore milled for 95 seconds. The calculation of the t-test values and comparison of these to standard t-values at a 95% probability (Skoog et al., 2004) proved that the differences in the mean pellet compressive strengths between these two data-sets were meaningful. However, for pellets prepared from ore milled for 120 seconds, there was no meaningful difference in the compressive strengths of the pellets. It can therefore be stated with a high level of confidence that oxidative sintered pellets prepared from UG2 ore are likely to have a similar or better compressive strength compared to pellets prepared from metallurgical-grade chromite ore.
Abrasion strength comparison
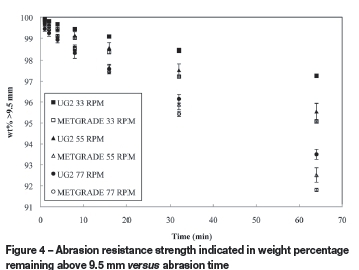
The abrasion strengths at different rotational drum speeds for oxidative sintered pellets prepared from UG2 and metallurgical-grade chromite ore are presented in Figure 4. All of these pellets were prepared from ore milled for 120 seconds in the Siebtechnik mill. It is evident from this data (Figure 4) that the abrasion strength of the UG2 oxidative sintered pellets was better than that of the oxidative sintered pellets prepared from metallurgical-grade chromite ore.
Microstructural analysis
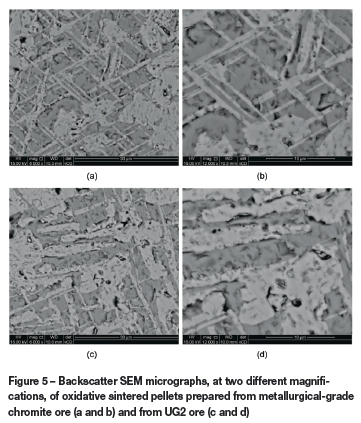
In order to assess why the compressive and abrasion strengths of oxidative sintered pellets prepared from UG2 ore were in general better than those of oxidative sintered pellets prepared from the metallurgical-grade chromite ore, microstructural analysis was performed. In Figure 5, SEM backscatter micrographs of polished sections of oxidative sintered pellets prepared from metallurgical-grade and UG2 chromite ore are presented at two magnifications. All these pellets were prepared from ore milled for 120 seconds with the Siebtechnik mill. From the micrographs, it is evident that the oxidative sintered pellets prepared from both case study ores had a crystalline structure. However, the pellets prepared from the metallurgical-grade chromite ore had a much finer and better defined crystalline structure than that of the pellets prepared from UG2 ore.
According to the Hall-Petch law (Equation [1]), structures with smaller grain size withstand a higher force before breaking (DolTPoMS, 2013).

where ay is the tensile yield stress, d is the grain diameter, ai is the intrinsic yield stress, and k is a constant for a particular material.
If the Hall-Petch law is applied, the pellets prepared from metallurgical-grade chromite would be expected to be stronger. This was clearly not the case (Figures 3 and 4). However, the Hall-Petch law applies only to grain structures of materials with the same composition. As indicated in Table II, the chemical and crystalline characteristics of the two case study ores differ. It is therefore unlikely that this law can be applied.
SEM secondary electron micrographs of the areas shown in Figure 5 are presented in Figure 6. In contrast to SEM backscatter micrographs (Figure 5), secondary electron micrographs (Figure 6) give a topographic perspective. Since these figures illustrate polished sections of both pellet types, limited depth perspective information can be derived from Figure 6. However, it seems as if the crystalline structure in the oxidative sintered pellets prepared from the metallurgical-grade chromite is better defined (Figures 6(a) and 6(b)) than the crystalline structure in the pellets prepared from the UG2, which blends into the general matrix (Figures 6(c) and 6(d)). This indicates that the crystals in the oxidative sintered pellets prepared from the metallurgical-grade chromite constituted a well-defined separate phase from the matrix, while in the pellets prepared from the UG2 ore, the crystals had assimilated better into the general matrix. The better assimilation of the crystalline growth into the matrix of the pellets prepared from the UG2 ore could at least partially explain why this type of pellet was stronger than that prepared form metallurgical-grade chromite (Figure 3 and 4).
X-ray mapping of elemental distribution in the two pellet types is presented in Figure 7. It is evident that the Mg distribution correlated best with the crystalline structures observed (Figures 5 and 6), with low Mg content in the crystals compared to the general matrix. Although not that well-defined, Fe was enriched in the crystals observed. Kapure et al. (2010) also observed Fe, in the form of the sequioxide phase (Fe2O3), precipitated on the surface of chromite grains in a typical Widmanstatten pattern, after pre-oxidation of chromite ore. However, in the current study both types of oxidative sintered pellet exhibited similar elemental distributions, and Fe and Mg distribution can therefore not be used to explain the differences in pellet strengths observed (Figures 3 and 4). Further research should be undertaken to investigate the possibly link between elemental distribution and the strength of oxidative sintered pellets prepared from metallurgical-grade chromite and UG2 ores.
Milling energy requirements
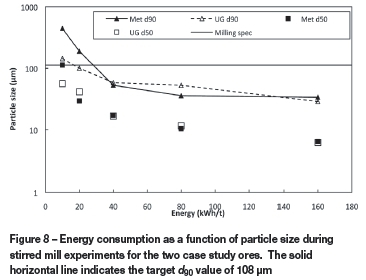
In Figure 8, the energy input measured during the stirred mill tests is presented as a function of particle size, i.e. d90and d50, for both case study ores. The targeted particle size, i.e. d90 of 108 pm, is indicated as a solid horizontal line. From these results, it is evident that the case study UG2 ore required 13 kWh/t less energy than the metallurgical-grade chromite to reach the target particle size. This is plausible, since the particle size distribution of the as-received UG2 ore was substantially finer than that of the as-received metallurgical-grade chromite (Table I). Although Cramer et al. (2004) mentioned that less energy is required to mill UG2 ore than metallurgical-grade ore, this advantage has not yet been quantified in the public peer-reviewed domain. With electricity costs rising rapidly in South Africa (Kleynhans et al., 2012) and electricity being the single largest cost component in FeCr production (Daavittila et al., 2004), this possible electricity saving during milling prior to pelletization could be significant for large FeCr producers. For example, if a generic FeCr producer with a production capacity of 300 000 t FeCr per annum is considered and it is assumed that 2.1 t of oxidative sintered pellets are consumed to produce 1 t FeCr, it can be calculated that approximately 4.15 million South African rands can be saved if the case study UG2 ore was used exclusively instead of the case study metallurgical-grade chromite ore. This calculation is based on an electricity unit cost of R0.523 per kWh (Eskom, 2011).
Conclusions
The results presented for the case study ores prove that the compressive strength of pellets prepared from the UG2 ore was the same, or better, than that of pellets prepared from the metallurgical-grade chromite. The UG2 pellets also had superior abrasion strength. It can therefore be stated that the use of UG2 ore, instead of metallurgical-grade chromite ore, is unlikely to result in the formation of additional fines (e.g. less than 6 mm) during material handling and feeding into submerged arc furnaces (SAFs). Only limited fine materials can be accommodated in SAFs, since fines can lead to unstable operation, as well as increased safety risks and equipment damage. SEM backscatter, as well as secondary electron and elemental X-ray mapping, indicated that the cured oxidative sintered pellets prepared from both the case study ores had developed a relatively well-defined crystalline structure. The crystalline structure of the pellets prepared from the UG2 ore was, however, better assimilated into the matrix, which could explain its general superior strength. The results further prove that less energy was required to mill the case study UG2 to the desired particle size, which can lead to substantial cost savings.
The results of this investigation can be used by South African FeCr producers to better quantify the advantages or disadvantages associated with using UG2 ore. Considering the size of the South African FeCr industry, decisions impacting the local FeCr industry also have an influence on the global FeCr market. Additionally, substantial volumes of UG2 ore are currently being exported for FeCr production outside South Africa. Therefore, the results can also assist these producers to contextualize the use of South African UG2 ore.
Acknowledgements
The authors wish to thank Glencore Alloys for financial assistance and Mintek for the use of their stirred mill.
References
Beukes, J.P., Dawson, N.F., and Van Zyl, P.G. 2010. Theoretical and practical aspects of Cr(VI) in the South African ferrochrome industry. Journal of the Southern African Institute of Mining and Metallurgy, vol. 110. pp. 743-750. [ Links ]
Beukes, J.P., Van Zyl, P.G., and Ras, M. 2012. Treatment of Cr(VI) containing wastes in the South African ferrochrome industry - a review of currently applied methods. Journal of the Southern African Institute of Mining and Metallurgy, vol. 112. pp. 347-352. [ Links ]
Cramer L.A., Basson, J., and Nelson, L.R. 2004. The impact of platinum production from UG2 ore on ferrochrome production in South Africa. Journal of the South African Institute of Mining and Metallurgy, vol. 104, no. 9. pp 517-527. [ Links ]
Daavittila, J., Honkaniemi, M., and Jokinen, P. 2004. The transformation of ferrochromium smelting technologies during the last decades. Journal of the South African Institute of Mining and Metallurgy, October. pp. 541-549. [ Links ]
DolTPoMS. 2013. University of Cambridge. http://www.doitpoms.ac.uk/tlplib/mechanical-testing/grainsize.php [Accessed 7 July 2013]. [ Links ]
ESKOM. 2011. Eskom retail tariff adjustment for 2011/2012. http://www.eskom.co.za/content/priceincrease2011.pdf [Accessed 13 october 2011]. [ Links ]
G&W Base (Pty) Ltd. 2013. Bentonite Ocean MD. http://www.gwbase.co.za/pdfs/Pel-Bond.pdf [Accessed 16 July 2013]. [ Links ]
Kapure, G., Tathavadkar, V., Rao, C.B., Rao, S.M., and Raju, K.S. 2010. Coal based direct reduction of peroxidised chromite ore at high temperature. Proceedings of the 12 th International Ferro-Alloys Congress (INFACON XII). Helsinki, Finland, 6-9 June 2010. pp. 293-301. [ Links ]
Kleynhans, E.L.J., Beukes, J.P, Van Zyl, P.G, Kestens, P.H.I., and Langa, J.M. 2012. Unique challenges of clay binders in a pelletised chromite prereduction process. Minerals Engineering, vol. 34. pp. 55-62. [ Links ]
Murthy, Y.R., Tripathy, S.K., and Kumar, C.R. 2011. Chrome ore beneficiation challenges and opportunities - a review. Minerals Engineering, vol. 24, no. 5. pp. 375-380. [ Links ]
Niemela, P., Krogerus, H., and Oikarinen, P. 2004. Formation, characterization and utilization of CO-gas formed in ferrochrome smelting. INFACON X, Proceedings of the Tenth International Ferroalloys Congress: Transformation through Technology, Cape Town, South Africa, 1-4 February 2004. South African Institute of Mining and Metallurgy, Johannesburg. pp. 68-77. [ Links ]
Neizel, B.W., Beukes, J.P, Van Zyl, P.G, and Dawson, N.F. 2013. Why is CaCO3 not used as an additive in the pelletised chromite pre-reduction process? Minerals Engineering, vol. 45. pp. 115-120. [ Links ]
Schneider, T. and Jensen, K.A. 2008. Combined single-drop and rotating drum dustiness test of fine to nanosize powders. Annals of Occupational Hygiene, vol. 52, no. 1. pp. 23-34. [ Links ]
SENTULA. 2013. Nkomati Anthracite (Pty) Ltd. http://www.sentula.co.za [Accessed 18 July2013]. [ Links ]
Singh, V. and Rao, S.M. 2008. Study of the effect of chromite ore properties on pelletisation process. International Journal of Mineral Processing, vol. 88. pp. 13-17. [ Links ]
Skoog, D.A, West, D.M, Holler, F.J., and Crouch, S.R. 2004. Fundamentals of Analytical Chemistry. 8th edn. (International Student Edition). Thomson Learning, Brooks/Cole. 155 pp. [ Links ]
Xiao, Z. and Laplante, A.R. 2004. Characterizing and recovering the platinum group minerals - a review. Minerals Engineering, vol. 17. pp. 961-979. [ Links ]
Paper received Nov. 2014
Revised paper received Feb. 2015
















