Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 no.8 Johannesburg Ago. 2015
http://dx.doi.org/10.17159/2411-9717/2015/v115N8A5
Smelting of calcined basic nickel carbonate concentrate in a 200 kW DC arc furnace
M. Abdellatif
Mintek, Randburg
SYNOPSIS
Calcined basic nickel carbonate (BNC) concentrate was smelted in a pilot-scale DC arc furnace to produce a nickel metal. The furnace was continuously operated for 12 days (24 hour/day), during which twelve different smelting conditions were investigated, with the major variables being reductant type and feed rate, flux composition and addition, and BNC feed rate. The 200 kW DC arc furnace was operated at power levels between 110 and 165 kW and at a total feed rate of 78 to 96 kg/h, resulting in an average slag and metal tapping temperature of about 1650°C. A total of 7.2 t of BNC were smelted, producing about 5.44 t of nickel metal and 2.94 t of slag. Nickel recoveries of 96.4% and higher were achieved, and the slag nickel content was as low as 0.1%. The major impurities in the metal were iron (mostly from oxygen lancing) and carbon. The calculated feed carry-over was less than 0.85% and the graphite electrode consumption was between 2.8-3.3 kg/MWh.
Keywords: nickel smelting, DC arc furnace, calcined basic nickel carbonate, BNC
Introduction
One of the most distinctive advantages of DC open-arc smelting is the ability to process fine raw materials without any major issues with regard to the operability of the furnace, metal recovery, and metal quality. This has been demonstrated in Mintek's DC arc pilot plant facilities over a period of about three decades (Curr et al., 1983). The materials that have been processed include laterite (Kotze, 2002), chromite fines (Curr et al., 1983), manganese fines (Lagendijk et al., 2010), PGM concentrates (Shaw et al., 2013), electric arc furnace (EAF) dust (Denton et al., 2005; Abdellatif, 2002a) and stainless steel (SS) dust (Denton et al., 2005; Abdellatif, 2002a; Goff and Denton, 2004), and petroleum fly ash (Abdellatif, 2002b). The top particle size of such materials can vary from a few millimetres (e.g. laterite) to micrometres (EAF and SS dust). Dusting, whether due to physical carryover of feed or to the arc side reactions, has been proven to be a non-issue. As such, the applications of DC open-arc furnaces have the potential to be extended to other raw materials such as particulate nickel oxide, which has a particle size much less than 100 μm.
Nickel oxide is typically produced from laterite ore by sulphuric acid leaching, purification, and precipitation as basic nickel carbonate (BNC) (Rhamdhani, Jak, and Hayes, 2008) or as mixed hydroxide precipitate (MHP) (Mackenzie, Virnig, and Feather, 2006), and finally calcining at moderate temperatures (800-1200°C). The NiO product is very fine, with an average particle size in the range of 10-20 μm and a NiO content of more than 99%.
Nickel oxide can be reduced to nickel metal by electrolysis (Moskalyk and Alfantazi, 2002), hydrogen reduction (Agrawal et al., 2006), or by the carbonyl process (Terekhof and Emmanuel, 2012). An alternative approach would be to smelt the NiO in a DC open-arc furnace in the presence of slag fluxes and a carbonaceous reducing agent. This alternative might offer faster reaction kinetics, and thus a smaller processing unit, as compared with hydrogen or CO reduction. In addition, it provides almost prompt metal separation from the slag, which allows the metal to be cast as required. The DC option may offer lower capital costs in comparison to the electrolytic process, as well as minimizing environmental pollution. This approach was investigated using Mintek's 200 kW DC arc facility. The results of the investigation are summarized in this paper.
DC arc pilot plant
The 200 kW DC arc pilot plant consists of a DC power supply, refractory-lined furnace, and an off-gas treatment system (Figure 1). The furnace comprises a refractory-lined cylindrical steel shell, a domed base, and a conical roof. The furnace shell has an unlined internal diameter of 980 mm and is provided with water-spray cooling. The conical roof contains two ports for feeding and a central port for a 100 mm graphite electrode (cathode). The roof is cooled by means of pressurized water panels.
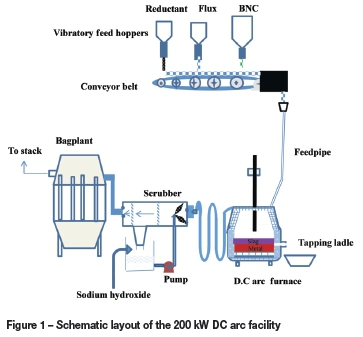
The furnace shell was lined with magnesia bricks, giving an internal diameter of about 670 mm. The base and the roof were lined with alumina castable.
The return electrode (anode) consists of several steel rods built into the hearth refractories. These rods are connected at their lower ends to a steel plate attached to the hearth dome, which is connected to the anode busbars.
The feed system comprised three feeders: the primary BNC feeder, the flux feeder, and a reductant feeder. Each hopper was equipped with load cells and a variable speed vibratory feeder in order to enable a controlled feed rate to be delivered to the furnace. The feed rate control was linked to the Delta V control and data acquisition system.
The power supply consisted of an 11 kV vacuum breaker, two isolators, two contactors, two transformers, and two 5 kA DC thyristor rectifiers. The gas extraction system comprised trombone coolers, a scrubber, a reverse-pulse bag filter, a fan, and a stack.
Experimental procedure
The initial warm-up period involved melting a 75 kg nickel heel, using cathode-grade nickel billets supplied by Insimbi Alloy Supplies, and charging two batches of raw materials into the furnace. The various feed components were then fed at the target values, and the power set-point adjusted to match the total feed rate and energy loss set-points. Feeding continued for between 1.5 and 3.0 hours, depending on the batch mass and the total feed rate. The metal and slag were then tapped into refractory-lined steel ladles by oxygen lancing. After initial cooling, the ladles were weighed and removed for further cooling and subsequent metal/slag separation, weighing, and storage.
The metal and slag temperatures were measured during tapping using an infrared pyrometer. Spoon samples were taken from both the metal and the slag stream. The slag samples were prepared for chemical analysis by crushing to about -15 mm, visually separating the metallic inclusions, and pulverizing to -75 pm. The metal samples were obtained by taking drill shavings using either a titanium or stainless steel drill bit.
The entrained solids in the furnace off-gas were captured in a scrubber unit using 1% NaOH solution. The scrubber slurry was removed every 24 hours, sampled, weighed, and analysed. Very little baghouse dust was recovered throughout the test work. When available, the dust was weighed, sampled, and analysed.
Samples of the various products (and the feed components) were chemically analysed using an ICP-OES method. Carbon and phosphorous were determined using a Leco technique.
The flux was prepared by mixing weighed quantities of the components using a Jones mixer. All operational data was monitored and recorded every two seconds on a PLC datalogging system.
The entire test work was carried out as one continuous campaign which lasted for 12 days (24 hours a day), and comprised 12 smelting conditions (Table I), in which the main variables were flux composition and addition rate, type and feed rate of reducing agent, and the total feed rate. The smelting conditions were guided by thermodynamic calculations carried out using the Pyrosim modelling software (Jones, 1987).
Raw materials
The raw materials employed in the test work consisted of eight different components. The nickel source was calcined basic nickel carbonate (BNC) concentrate. The average analysis of the BNC is shown in Table II. The particle size averaged about 10 μm, with a top size of about 40 μm.
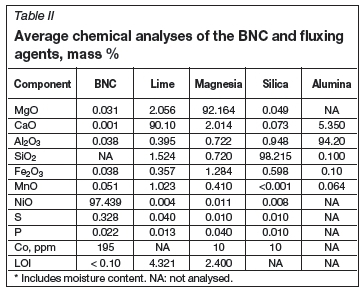
Three different reducing agents were utilized, namely petroleum coke (referred to as Sascarb), anthracite coke, and metallurgical coke (Table III). Sascarb is a relatively pure carbon source (>99% C) and has a narrow particle size range of 3-5 mm. The Sascarb was acquired from Sasol. Anthracite coke has a relatively low fixed carbon content (79.7% C), with a particle size of -10 mm. The metallurgical coke analysed at about 84% fixed carbon and had a particle size of less than 10 mm.
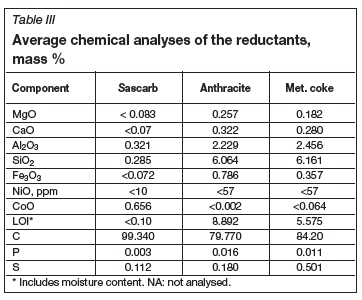
The flux used in Conditions 1-6 (Table I) consisted of lime, magnesia, and silica (Table II), where the target basicity ratio was kept at 1.2-1.0 (defined as (CaO + MgO)/SiO2). The particle size of both lime and magnesia was -5 mm, while that of silica was between 2 mm and 10 mm. In Conditions 7-12, alumina was added to the flux mixture with a target A12O3 content in the slag of 20%, while keeping the basicity ratio at 1.2-1.0 (defined as (CaO + MgO)/ (SiO2 + Al2O3)). The alumina used had a particle size of -10 mm.
Results
Slag analyses
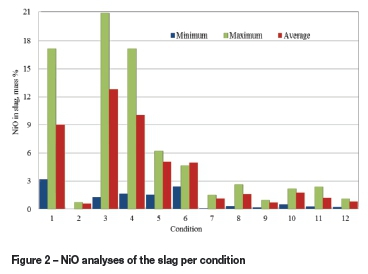
The weighted average slag analysis for the various conditions is presented in Table IV (see also Figure 2 for nickel oxide range of analysis). With the flux-1 recipe, the nickel content in the slag remained relatively high for the first six conditions, ranging from about 0.60% to 10.1% NiO, although in certain taps it dropped to below 0.5%. (Condition 2 is excluded from the analysis as it consisted of only two taps). This may be attributed to several factors.
> Metallic inclusions in the samples. This was evident from the analysis of cleaner samples taken from the ladle, which had a significantly lower nickel content. (Ni analyses of certain spoon sample taken during tapping were 50% higher than analyses of the corresponding clean samples taken from the ladle.) Incomplete metal-slag separation in the furnace was attributed largely to the viscous and sticky slag
> The average furnace tapping temperatures were generally lower than those achieved in subsequent conditions
> Possibly insufficient reductant.
Replacing Sascarb with anthracite while keeping the fixed carbon addition constant (Condition 3) did not have a major impact on the nickel content in the slag, as compared to Condition 1. However, if the results of taps 1-3 (warm-up period) are excluded from Condition 1, the use of anthracite appears to decrease the slag nickel content in certain taps to about 1.0%, compared to a low of 4.3% realized in Condition 1. In Conditions 4-6, the Sascarb addition was gradually increased from 12% to 14% of the BNC mass, compared to 11.2% in Condition 1. This resulted in a significant decrease in nickel oxide content in the slag, to an average of about 4% in Conditions 5 and 6. The minimum values achieved in certain taps during these three conditions were not significantly different, ranging from 1.22 to 1.86% NiO.
Condition 7 represents the start of the flux-2 recipe (addition of alumina). The Sascarb addition was kept at 14% (the same as for Condition 6). The resulting slag had the lowest average NiO content (about 0.88%) thus far. Further increase in the reductant addition to 16% (Condition 8) yielded a slag containing less than 0.5% NiO in certain taps.
With lower flux addition (30% of the BNC - Condition 9), nickel oxide in the slag averaged 0.56%, and reached a level of 0.13% in more than one tap. A further decrease in the flux addition in Condition 10 appears to have resulted in an increase in the NiO content (1.38% average, with the lowest being 0.41%).
Metallurgical coke was employed as the reducing agent in Condition 11 (16.8% addition to give a similar fixed carbon addition as in Condition 7). Simultaneously, the flux-2 addition was increased to 40% of the BNC mass. The slag produced contained between 0.2 and 2.1% NiO. These results suggest that the metallurgical coke was somewhat more effective than Sascarb in reducing the nickel oxide. The feed recipe for Condition 12 was similar to that for Condition 11, but the feed rates were increased by 20%. The nickel oxide content in the slag did not change significantly from that obtained in Condition 11, averaging about 0.6%, with a minimum value of 0.18%.
The slag from certain taps had a relatively high FeO content of up to 13.8%. This was most likely due to excessive lancing, which was required on a few occasions. The average total slag composition exceeds 100% in most of the conditions (Table IV). This might be related to metallic inclusions, as well as analytical errors.
Metal analyses
The weighted average chemical analyses of the metal are shown in Table V. The nickel produced in Conditions 1-6 (flux-1 recipe) analysed at between about 0.9% and 4.5% impurities, the main impurity element being iron. This is believed to be due to oxygen lancing, in addition to the iron present in the feed materials. The second major impurity is cobalt at about 0.10-0.34%. The carbon content in the metal varied between 0.01% and 0.42% except for Condition 6, where it increased to about 1.0% as a result of the increased carbon addition.
Condition 7 produced a cleaner metal in terms of iron content, as lancing was very moderate throughout most of the taps. However, the carbon content averaged about 2.0%. Further increase in the reductant addition (Condition 8) yielded lower metallic impurity levels, totalling about 3.3%. In this condition the iron content dropped to about 0.2%. In Condition 9 (30% flux-2 and 14% Sascarb), similar quality metal to that of Condition 8 was produced, with only a marginal increase in both carbon and sulphur contents.
Carbon and iron were the major impurity elements in the nickel produced in Condition 10, averaging about 1.8% and 1.6% respectively. Similar quality metal was produced in Condition 11, where metallurgical coke was employed, except for the iron and carbon contents, which averaged about 1.0% and 2.3% respectively. With a higher smelting rate during Condition 12, iron content increased to about 1.4%, while the level of carbon decreased to 1.4% compared to 2.3% Condition 11.
Fume analyses
The major component in the fume product is nickel oxide (about 48%, Table VI), making it suitable for recycling to either the furnace or the calciner, depending on the sulphur content and other impurities. Semi-quantitative XRD analysis suggested that the crystalline phases are 32% Ni, 51% NiO, 12% Ni3S2, and 5% CaCO3.
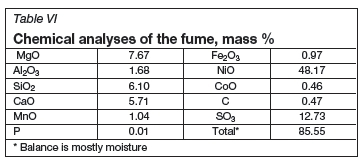
Scrubber products
The normalized sludge analysis appears to be similar to that of the fume, particularly with regards to nickel oxide and sulphur contents (Table VII). Therefore, it might be possible to recycle the sludge to the calciner in order to remove the moisture and to minimize the sulphur content before feeding it to the furnace.
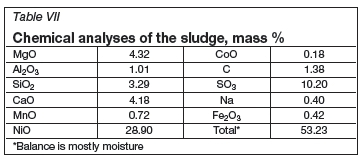
Several samples were taken from the effluent stream. The nickel content was well below 2 ppm in all of these samples, and therefore the effluent stream required only neutralizing before disposal.
Feed carry-over
The proportion of the feed materials that escaped the furnace with the off-gas was calculated via two approaches. The first assumed that all the fume and scrubber sludge (dry basis) were unreacted materials and therefore represented the total losses. This approach results in a feed carry-over of about 0.85%. The second approach was based on the calcium content in the fume and scrubber sludge relative to that in the feed materials. Specifically, the feed carry-over was calculated from the following relationship:
Carry-over = Mass% Ca in fume χ Fume masses χ 100% /(Mass% Ca in feed χ Total mass fed)
This approach resulted in a feed carry-over of about 0.5%, which is significantly less than that of the first approach. Nevertheless, both methods suggest that the feed losses to the off-gas were very low, particularly given the scale of operation and the particle size of the feed components.
Overall mass balance
The overall mass balance for the entire test campaign shows an accountability of about 103%, or about 308 kg of excess products, as compared to the masses fed (Table VIII). This is believed to be related to the CO/CO2 mass ratio of 1.22/1.0 as predicted by Pyrosim (Jones, 1987), oxygen lancing, which introduced more than 368 kg of iron into the furnace products, and refractory erosion.
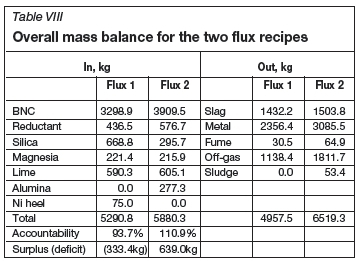
For the first flux recipe, the overall accountability was 93.7%, representing a deficit of about 333 kg. This is mostly slag and metal build-up in the furnace that could not be tapped at the end of this period. Conversely, the flux-2 recipe exhibited an overall accountability of about 111%, or a surplus of about 640 kg.
Nickel mass balance
For the flux-1 recipe, the nickel accountability was about 90.3%, compared to about 101.0% for the flux-2 recipe. Inclusion of the metal digout in the flux-2 recipe period is believed to be the major reason for this. Based on mineralogical examination of three slag samples, metallic nickel in the slag ranged from about 2.1% to 5.4% (mass basis), averaging about 4.23% in the samples tested. This is equivalent to about 125 kg of metal trapped in the slag. Taking this into account, the overall nickel accountability improves to over 98.2%.
Nickel recoveries averaged about 96.4% and 99.5% for the flux-1 test work and 88.8 and 99.7%, for flux-2, for metal and slag masses and analyses respectively.
Energy balance and electrode consumption
Based on the measured heat losses and the total energy input, the furnace thermal efficiency averaged about 36.5% in Conditions 1-6. It dropped to about 29.5% in Conditions 7-12, possibly due to refractory erosion. It should be noted that the furnace power intensity averaged about 300 kW per square metre of furnace hearth area during the first six conditions. It then increased to 390 kW/m2 for the rest of the test work, except for Condition 12 where it averaged about 450 kW/m2.
The electrode consumption was 3.3 kg/MWh in the first six conditions. Longer arc length, and thus lower current density, and more steady operation during the flux-2 test work contributed to the almost 38% drop in electrode consumption (2.8 kg/MWh), as compared to the flux-1 recipe.
Discussion
Although the nickel recovery during flux-2 test work was very high at fixed carbon additions of 14% of BNC or higher, it is believed that further optimization can be achieved. A larger and better sealed furnace may reduce air ingress, and thus carbon losses through oxidation. The carbon addition could be adjusted to control the carbon content of the metal and to increase the slag sulphur capacity, in addition to controlling the reduction of nickel oxide. These are somewhat contradictory objectives, but a balance may need to be found. Proper selection of the reducing agent and the flux components can help reduce the sulphur content in the metal (Pashkeev et al., 2011; Shankar, 2006; Ren, Hu, and Chou, 2013; Wang et al., 2009), and thus its refining requirements, if any.
Smelting of the BNC in the presence of a CaO-MgO-SiO2 slag (flux-1 test work) was somewhat difficult and resulted in slags with relatively high nickel contents. However, increased fixed carbon additions tended to increase nickel recovery, as is shown by the drop in the average nickel content in the slag. Nickel losses to the slag are believed to be affected by metallic inclusions, as the slag tended to be sticky. The formation of high-melting compounds (carbides) could have affected the flowing characteristics of the slag and hindered the settling of the metal.
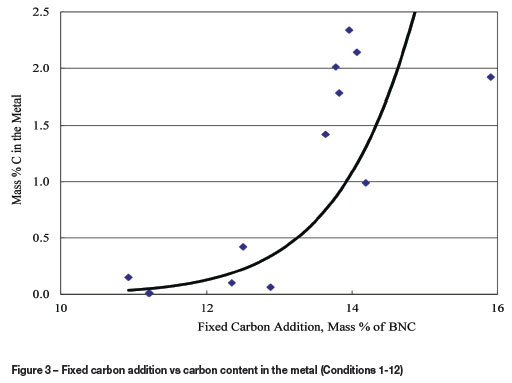
With flux-2 recipe there did not appear to be a clear relationship between the average nickel content of the slag and the fixed carbon addition within the range studied. The lowest carbon addition during this period (about 13.6%) was sufficient to lower the nickel levels to below 1.0%, on average. Any extra carbon added is believed to have resulted in the reduction of other metal oxides, and more importantly in the accumulation of reductant in the furnace. In addition, elevated reductant additions tended to increase the carbon content in the product metal (Figure 3).
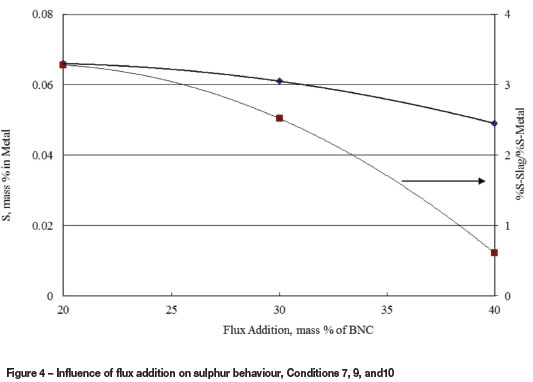
In addition to enhancing nickel extraction, higher carbon additions were aimed at lowering the oxygen potential in order to increase the sulphur capacity of the slag, and therefore decrease the sulphur content of the metal. The results, however, did not show a strong relationship between the average sulphur content in the metal and fixed carbon addition. As indicated previously, the slag/metal ratio, slag depth, slag composition, and operating temperature were not constant throughout the test work, which would account for the apparent lack of such correlation. For example, when the results of Conditions 7, 9, and 10 are examined separately (flux additions of 40, 30, and 20% respectively), it could be concluded that increased flux addition tended to lower the sulphur content of the metal. At the same time, the mass ratio (% Sslag/% Smetal) tended to drop with increased flux addition, mostly due to dilution effects (Figure 4). Overall, and based on the sulphur mass balance, about 19.8% and 20.4% of the sulphur in the feed reported to the slag and metal respectively, with the balance being in the fume, sludge, and the scrubber effluent. The sulphur accountability is about 104%.
Conclusions
Particulate BNC concentrate was successfully smelted in a 200 kW DC arc furnace to produce a metal containing 96% Ni and higher. Two slag recipes were employed. The first consisted of 40% CaO, 15% MgO, and 45% SiO2. Nickel recoveries were acceptable and averaged about 96.4%, with the slag containing 8.4% NiO on average. The recovery of the metal was improved with increased reductant additions and higher operating temperatures.
Nickel recovery to the metal phase increased to 99.7% when the second slag recipe was used. The produced slag (40% CaO, 15% MgO, 25% SiO2, and 20% A2O3) contained less than 1% NiO on average. Nickel recovery did not vary significantly, regardless of the type of reductant, total feed rate, or the amount of flux used.
Sulphur retention in the slag appears to have been influenced by the slag composition and, to a lesser extent, by the reductant addition. Carbon content in the metal tended to increase with higher reductant additions, regardless of the slag composition or the nature of the reductant.
Despite the very fine particle size of the BNC, feed carry over was well below 0.9% throughout the test work. The fume contained about 40% NiO, and can be easily recycled to the calcining step in order to minimize the sulphur content and to drive off the moisture.
References
Abdellatif, M. 2002a. Fundamentals of zinc recovery from metallurgical waste in the Enviroplas process. Minerals Engineering, vol.15. pp. 945-952. [ Links ]
Abdellatif, M. 2002b. Recovery of vanadium and nickel from petroleum flyash. Minerals Engineering, vol. 15. pp. 953-96. [ Links ]
Agrawal, Α., Kumar, V., Pandey, B.D., and Sahu, K.K. 2006. A comprehensive review on the hydrometallurgical process for the production of nickel and copper powders by hydrogen reduction. India Materials Research Bulletin, vol. 41. pp. 879-892. [ Links ]
Curr, T.R., Barcza, N.A., Maske, K.U., and Mooney, J.F. 1983. The design and operation of transferred-arc plasma systems for pyrometallurgical application. 6th International Symposium on Plasma Chemistry (ISPC-6), Montreal, Canada, July 1983. [ Links ]
Denton, G.M., Barcza, N.A., Scott, P.D., and Fulton, T. 2005. EAF dust processing. John Floyd International Symposium on Sustainable Developments in Metal Processing, Melbourne, Australia, 3-6 July 2005. Nilmani, M. and Rankin, W.J. (eds). Australasian Institute of Mining and Metallurgy. pp. 273-283. [ Links ]
Goff, T.J and Denton, G.M. 2004. Direct smelting of stainless steel plant dust. INFACON X. Proceedings of the 10th Internatonal Ferro-Alloys Congress, Cape Town, South Africa, February 2004. pp. 687-691. [ Links ]
Jones, R.T. 1987. Computer simulation of pyrometallurgical processes. APCOM '87. Proceedings of the Twentieth International Symposium on the Application of Computers and Mathematics in the Minerals Industries, Johannesburg, South Africa. Vol. 2: Metallurgy. South African Institute of Mining and Metallurgy, Johannesburg. pp. 265-279. [ Links ]
Kotze, I.J. 2002. Pilot plant production of ferronickel from nickel oxide ores and dust in a DC arc furnace. Pyrometallurgy 02, Mount Nelson Hotel, Cape Town, South Africa, 11-12 March 2002. [ Links ]
Lagendijk, H., Xakalashe, B.S., Ligege, T., Ntikang, P., and Bisaka, K. 2010. Comparing manganese ferroalloy smelting in pilot scale AC and DC submerged arc furnaces. INFACON XII. Proceedings of the 12th International Ferro-Alloys Congress, Helsinki, Finland, 6-9 June 2010. pp. 497-507. [ Links ]
Mackenzie, M., Virnig, M., and Feather, Α. 2006. The recovery of nickel from high-pressure acid leach solutions using mixed hydroxide product-LIX(r) 84-INS technology. Minerals Engineering, vol.19, no. 12. pp. 1220-1233. [ Links ]
Moskalyk R.R. and Alfantazi, A.m. 2002. Nickel laterite processing and electrowinning practice. Minerals Engineering, vol. 15. pp. 593-605. [ Links ]
Pashkeev, I. Yu., Pashkeev, A.I., and Vlasov, V.N2011. Sulfur distribution between a slag and a metal in melting of carbon ferrochromium. Russian Metallurgy (Metally), vol. 2011, no. 12. pp. 1138-1140. [ Links ]
Rhamdhani, Μ.Α., Jak, E., and Hayes, P.C. 2008. Basic nickel carbonate; Part I. Microstructure and phase changes during oxidation and reduction processes. Metallurgical and Materials Transactions B, vol. 39B. pp. 218-228. [ Links ]
Shaw, Α., De Villiers, L.P.vS., Hundermark, R.J., Ndlovu, J., Nelson, L.R., Pieterse, B., Sullivan, R., Voermann, N., Walker, C.. Stober, F., and McKenzie, A.D. 2013. Challenges and solutions in PGM furnace operation: high matte temperature and copper cooler erosion; Journal of the Southern African Institute of Mining and Metallurgy, vol. 113, no. 3. pp. 251-26 [ Links ]
Terekhov, D.S. and Emmanuel, N.V. 2012. Direct recovery of nickel and iron from laterite ores using the carbonyl process. Proceedings of Processing of Nickel Ores and Concentrates'12, Cape Town, South Africa, November 2012. [ Links ]
Shankar, Α. 2006. Sulphur partition between hot metal and high alumina blast furnace slag. Ironmaking and Steelmaking, vol. 33, no. 5. pp. 413-418. [ Links ]
Ren, Z-S., Hu, X-J., and Chou, K-C. 2013. Calculation and analysis of sulphide capacities for CaO-Al2O3-SiO2-MgO-TiO2 slags. Journal of Iron and Steel Research International, vol. 20, no. 9. pp. 21-25. [ Links ]
Wang, J-J., Guo, S-X., Zhou, L., and Li, Q. 2009. Slag for decopperization and sulphur control in molten steel. Journal of Iron and Steel Research International, vol. 16, no. 2. pp. 17-21. [ Links ]
Paper received Jan. 2014
Revised paper received May 2014














