Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 no.8 Johannesburg Ago. 2015
http://dx.doi.org/10.17159/2411-9717/2015/V115N8A4
Effects on entrainment of serpentines by hydrophobic flocs of ultra-fine copper-nickel sulphides during flotation
M. Tang; S. Wen; X. Tong
Department of Mineral Processing, Kunming University of Science and Technology, China
SYNOPSIS
Slime coating is one of the most common ways for serpentines to contaminate metallic mineral concentrates during traditional flotation of coarse sulphide particles. This could pose quite a complicated and challenging problem in the case of some types of low-grade and finely disseminated Cu-Ni ores bearing high serpentine contents. This is the case for the copper and nickel sulphides from the Yunnan Mine, China. Previous batch flotation tests of this ore resulted in satisfactory recoveries of 86.92% Cu, 54.92% Ni, and 74.73% Pt+Pd, and concentrate grades of 4.02% Cu, 3.24% Ni, and 76.61 g/t Pt+Pd. However, the MgO content in these concentrates was more than 19%. In the current study, microflotation tests and settling rate tests were introduced to investigate the effects of a combination of strong collectors (a 2:1 weight ratio of butyl xanthate and butyl ammonium dithophosphate) on entrainment of serpentines in metallic mineral concentrate, as well as visual observations of the concentrates in suspension using still photography. All test results indicated the presence of serpentines entrapped in the hydrophobic flocs that resulted from these collectors, even with the use of effective gangue depressants. These strong collectors are used to flocculate the ultra-fine sulphides by forming loose and 'fluffy' hydrophobic flocs. However, these hydrophobic flocs may also be able to load or entrap some serpentine slimes into the concentrate, and this entrained serpentine could be harder to remove by using depressants or intensified conditioning than serpentine slime coating on the particle surfaces.
Keywords: sulphide flotation, serpentine, hydrophobicity, ultra-fine, entrainment
Introduction
Serpentine minerals, which have the generalized composition (Mg,Fe)3Si2O5(OH)4, can be easy to crush and grind due to their convoluted and bent layered structures. These serpentines can hinder the enrichment of some metallic minerals and dilute their concentrates by entrainment during flotation. The resulting high pulp viscosities, slime coatings, and high content of dissolved ions adversely affect the flotation recoveries of copper and nickel sulphides, and high levels of MgO entrained in the concentrates lead to heavy penalties from smelters. Since the quality of nickel flotation concentrate depends heavily on its MgO content (generally less than 6-7% for No.1 or 2 grade nickel concentrate according to the standard requirement from National Non-ferrous Industry), reducing the entrainment of serpentine during flotation is becoming more and more pressing. Previous research has focused on the collectors and depressants that are commonly used in copper-nickel flotation, as well as their interaction mechanisms. The traditional collectors, like xanthate (Senior et al., 2005; Malysiak et cel., 2002; Bozkurt et cel., 1998), dithiophosphate (Wiese et al., 2006), or mixed/combined collectors (Glembotaskii, 1958; Lotter and Bradshaw, 2010; Wiese et al., 2008) are used to enhance the hydropho-bicity of copper or nickel sulphides during flotation. Lu et al. (2011) indicated that potassium amyl xanthate is adsorbed on a mixture of pyrite and serpentine, but that adsorption is minimal on serpentine alone in alkaline pulp. Feng et al. (2012) found that the longer the hydrocarbon chain of xanthate, the lower the Ni grade and recovery, indicating that serpentine entrainment is influenced significantly by the type of collector. These collectors might not affect the natural floata-bility of serpentine directly, as indicated by Edwards et al. (1980). However, they may possibly influence the entrapment of serpentine indirectly by forming hydrophobic flocs of ultra-fine metal values. Also, slime coating is a common way for serpentines to be incorporated into metallic mineral concentrates. However, according to some researchers (Wellhame et al., 1992; Bulatovic, 1999; Chen et al., 1999), slime coatings can be reduced or removed from nickel mineral surfaces through strong and lengthy agitation. A possible additional mechanism for serpentine entrainment during the flotation of this type of ultra-fine ore, other than water recovery or slime coating, is indicated by some unsatisfactory results, coupled with high content of MgO in concentrates, even when using efficient depressants like carboxymethyl cellulose (CMC) and guar gum combined with high-intensity agitation (Wellhame et al., 1992; Senior et al., 1994; Senior and Thomas, 2005). The flocculation behaviour of ultra-fine metallic values during flotation may have an important influence on the entrainment of serpentine.
Although the mechanism of serpentine entrainment into sulphide concentrates during coarse particle flotation by slime coating or water recovery is well established, the possible effects of hydrophobic flocs of fine sulphide particles resulting from strong collectors is not sufficiently understood. In this study, batch flotation tests, microflotation tests, and settling rate measurements were conducted with the object of investigating the indirect effects of strong combined collectors on the entrainment of serpentines during flotation of fine grained copper-nickel ore containing Pt and Pd from the Yunnan Mine, China, with particular emphasis on the role of the hydrophobic flocs of ultra-fine metallic sulphides resulted from the use of these collectors.
Experimental
Materials
The finely disseminated ore from Yunnan Mine, in China, assays about 0.15% Cu and 0.21%Ni, with minor amounts of Pt and Pd. The main sulphide minerals are chalcopyrite, penlandite, pyrrhotite, and violarite. The gangue minerals are dominated by serpentines, which account for 75% of the MgO. The platinum group metals (PGMs) in Yunnan ore are strongly associated with the copper-nickel sulphides, especially nickel sulphides.
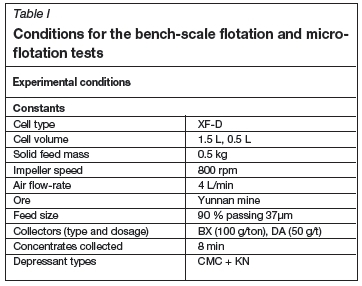
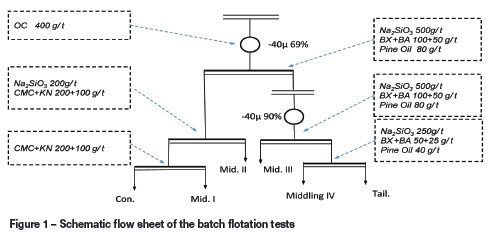
Samples of pure pentlandite and serpentine (>99%, -20 um) were purchased from Jinchuang Mine, China. The flotation reagents included butyl xanthate (BX), butyl ammonium dithophosphate (BA), sodium silicate, (carboxymethyl cellulose, CMC), and pine oil, which were purchased from Kunming Metallurgical Research Institute, Yunnan province, China, and KN and OC (our patented reagents), which are traditional reagents that have been modified or combined with other reagents. OC acts as an inorganic activator that can effectively activate oxidized pentlandite; its major component is copper sulphate, accompanied by other salts. KN is modified guar gum, which acts as a depressant in combination with CMC. The open-circuit flow sheet and experimental conditions for the batch flotation tests and microflotation tests are shown in Table I and Figure 1.
Methods
Batch flotation tests
To prepare the sample for the batch flotation tests, 500 g of the sample was ground in a ball mill (ΧΜΟ-67Φ240χ90 mm, from Zhuzhou Mining Equipment, China) for 10 minutes to a size of 80% -74 um. The flotation tests were conducted in a mechanical laboratory machine using a 1.5 L cell (XF-D) for the roughing and scavenging stages and a 0.5 L cell for cleaning. A mixture of BX and BA in a 1:2 ratio was used as the main collector. Sodium silicate was employed as dispersant of gangue minerals, and pine oil as frother. All the tests were conducted at natural pH (6.7). The rougher tails were re-ground, and cleaning and scavenging were conducted in the 0.5 L and 1.5 L cells for 5 and 8 minutes respectively. Suspensions of the final concentrates from the above tests were photographed at high resolution using an Olympus camera. The concentrate, as well as mixtures of middlings and tailing from the bench flotation tests, was filtered, dried, weighed, and analysed for Ni, Cu, and PGMs.
Microflotation tests and visual observation
A Hallimond tube was used for microflotation of pure pentlandite and serpentine. Measured weights of the pure minerals were washed with dilute acid, dried in a nitrogen atmosphere, then conditioned by the combined collectors (BX plus BA in a 1:2 ratio) at a chosen dosage, and floated at a suitable bubble size for 1 minute. The concentrate was collected, dried, and weighed, and suspensions photographed as for the batch flotation products.
Settling rate measurements
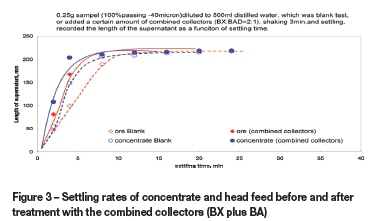
To prepare the samples for settling rate measurement, a head feed was ground to a size range of 100% passing 40 um, then dried and sampled. A concentrate sample from batch flotation tests was treated with Na2S as de-collector in boiling water for 1 hour, then filtered, dried, and sampled. A 150 g/t addition of BX plus BA in a weight ratio of 2:1 was made to both samples, which were then stirred for 3 minutes in the beakers before settling rate measurement. The settling rate tests were performed using a 250 mL graduated glass cylinder (280 mm high and 40 mm in diameter).
Results and discussion
Effects of combined collectors on entrainment of serpentines in batch flotation tests
The effects of dosage of the combined collectors (BX plus BA at 1:2 weight ratio) on nickel recovery and grade in concentrate from batch flotation tests are shown in Figure 2. A significant increase in Ni recovery to the concentrate (10% increase) was obtained by increasing the combined collector dosage from 90 g/t to 150 g/t, but at the cost of a sharp drop in the concentrate grade, from 0.92% Ni to 0.70% Ni. According to previous research (Bulatovic, 2003), strong collectors like xanthate can efficiently and selectively recover fine metallic sulphides, but there may be little effect on serpentines even at increased dosages. The much more hydrophobic flocs of the ultra-fine metal minerals resulting from higher dosages of these combined collectors may lead to a significant improvement in the recovery of Ni to concentrate, but their 'fluffy' structures may cause more serpentine slimes to be trapped in the concentrate, even when using the efficient depressants (CMC plus KN). It can also be seen from Figure 2 that both the recovery and grade of Ni in the concentrates deteriorated at combined collector additions of more than 150 g/t, indicating that increased collector dosages do not form more hydrophobic flocs, due to the limited number of the free-liberated metal minerals.

Effects of combined collectors on entrainment of serpentine - settling rate measurements
Figure 3 compares the settling rates of a head feed and a de-collector concentrate before and after treatment by the combined collectors (BX plus BA) at the same dosages that were used in the batch flotation tests. The data indicates that in the first 8 minutes, the settling rates of both the head feed and concentrate were significantly faster when treated with combined collectors. Furthermore, the settling rate of this concentrate (treated with the collectors) is much faster than the others, suggesting that the combined collectors increase the sizes of these fine metal mineral aggregates by forming large and fluffy hydrophobic flocs, thus substantially increasing their settling rates. However, much less serpentine was observed in supernatants from samples digested with BX plus BA than those without any collectors after the first 8 minutes. This indicates that using these collectors may promote the entrainment of serpentine due to the formation of many fluffy hydrophobic flocs of metal minerals as 'containers', which is in accordance with the results from batch flotation tests as shown in Figure 2. This suggests that most of the serpentine slime may report to the concentrate by becoming trapped in or coated on these hydrophobic flocs of ultra-fine metal minerals.
Effects on entrainment of serpentine by combined collectors - microphotographic measurements
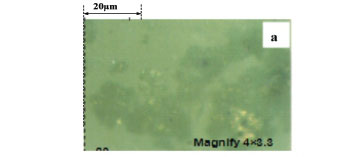
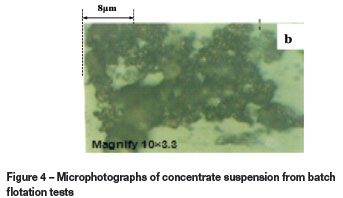
Figures 4a and 4b show photographs of suspensions of the concentrates from batch flotation tests. These images show some loose and fluffy hydrophobic flocs (dark colour) in suspension, coupled with some serpentine slimes (light grey colour) either by entrapment in or coating on these hydrophobic flocs. This indicates that both coating and entrapment may contribute to the entrainment of serpentine in concentrates. However, since the recoveries and grades of copper and nickel in these concentrates, as well as the MgO content, are only slightly affected by high-intensity conditioning (an increase in the stirring speed from 800 to 1600 r/min in an attempt to remove the slime coating), it is likely that entrapment makes a greater contribution to serpentine entrainment than coating. It is noteworthy that this mechanism of slimes coating on the relatively coarse (>37 um) sulphide particle surfaces might be more favourable for serpentine entrainment than the other due to very little hydrophobic flocs being formed by strong collectors. These results agree well with those illustrated in Figures 2 and 3.
Possible mechanisms of entrapment and entrainment of serpentine in concentrates
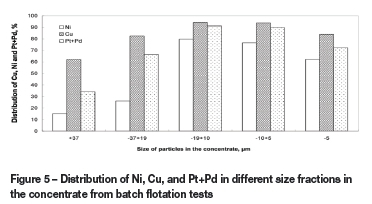
Figure 5 compares the recoveries of Cu, Ni, and Pt+Pd at different size fractions in the concentrate from batch flotation tests based on the flow sheet shown in Figure 1. High recoveries (>80% of Cu, Ni, and Pt+Pd) were obtained in the -37 um size fraction, particularly in the -20 um fraction, but the MgO content of the concentrate remained almost 19%. These results suggest that the ultra-fine copper and nickel sulphides in the ore samples can be effectively flocculated by using the strong combined collectors (BX plus BA) due to the formation of fluffy hydrophobic flocs that attach to the bubbles and report to the concentrate. However, the high serpentine content of this concentrate makes these results unsatisfactory. These collectors have little influence on the floatability of serpentine minerals, according to previous research by Lu et al. (2011). Based on the results from microphotographs and settling rate measurements, the possible mechanisms of serpentine entrainment may relate to both entrapment in and coating on these fluffy hydrophobic flocs of ultra-fine copper and nickel sulphides. An attempt to remove the entrapped and entrained serpentine by using intensified conditioning ( 1600 r/min for 30 minutes) before batch flotation resulted in a very slightly decrease of MgO grade in the concentrate. That indicates that serpentine entrapment in metal sulphide flocs may play much more important role than coating on the particles. Those results agree well with previous research (Wellhame et al., 1992; Senior et al., 1994; Senior and Thomas, 2005). Certainly, serpentine recovery by water recovery should not be ignored, but is probably less important than other mechanisms after using CMC and KN as depressants.
Conclusions
Based on the results of batch flotation and microflotation tests, the combined collectors (BX plus BA at a 1:2 weight ratio) can effectively flocculate the ultra-fine metal sulphide minerals, forming a certain amount of loose and fluffy hydrophobic flocs that attach to the bubbles. However, these flocs might correspondingly provide many potential spaces for serpentine entrainment into the concentrate by either entrapment in or coating on the flocs, resulting in high MgO contents (up to 19%) in the concentrate. The entrapment of serpentine slimes in the hydrophobic flocs may play an important role in contaminating the concentrate at a size range of less than 37 Mm. Entrapped serpentine slimes will be more difficult to remove that slimes coated onto sulphide particles, which can be removed by high-intensity conditioning or using efficient depressant/flocculants.
Acknowledgements
MT acknowledges the Wenbin Zhang's project on ultra-fine copper-nickel sulphides containing platinum metal groups in Yunnan, which had been completed. Financial support for this project was provided by the Natural Sciences Council of China. The authors also appreciate support from the Scholarship of Yunnan Science.
References
Bozkurt, V., Xu, Z., and Finch, J.A. 1998. Pentlandite/pyrrhotiite interaction and xanthate adsorption. International Journal of Mineral Processing, vol. 52. pp. 203-214. [ Links ]
Bulatovic, S.M. 1999. Use of organic polymers in the flotation of polymetallic ores: a review. Mineral Engineering, vol. 12. pp. 341-354. [ Links ]
Bulatovic, S.M. 2003. Evaluation of alternative reagent schemes for the flotation of platinum group minerals from various ores. Minerals Engineering, vol. 16. pp. 931-939. [ Links ]
Chen, G., Grano, S., Sobieraj, S., and Ralson, J. 1999. The effect of high intensity conditioning on the flotation of a nickel ore, paper 2: mechanisms. Mineral Engineering, vol. 12. pp. 1359-1373. [ Links ]
Edwards, C.R., Kipkle, W.B., and Agar, G.E. 1980. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. International of Journal of Mineral Processing, vol. 7. pp. 33-42. [ Links ]
Feng, B., Feng, Ο., Lu, Y., and Lv, P., 2012. The effect of conditioning methods and chain length of xanthate on the flotation of a nickel ore. Minerals Engineering, vol. 39. pp 48-50. [ Links ]
Glembotskii, A.A. 1958. The combined action of collectors during flotation. Tsvetnye Metally, vol. 4. pp. 6-14. [ Links ]
Lotter, N.O. and Brandshaw, D.J. 2010. The formulation and use of mixed collectors in sulfide flotation. Minerals Engineering, vol. 23. pp. 945-951. [ Links ]
Lu, Y., Zhang, M., Feng, Ο., Long, T. L., Ou, L., and Zhang G. 2011. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Transactions of Nonferrous Metals Society of China, vol. 21. pp. 208-213. [ Links ]
Malysiak, V., O'Connor, C.T., and Ralston, J. 2002. Pentlandite-feldspar interaction and its effect on separation by flotation. International Journal of Mineral Processing, vol. 66. pp. 89-106. [ Links ]
Senior, G.D., Shannon, L.K., and Trahar, W.J. 1994. The flotation of pentlandite from pyrrhotite with particular reference to the effects of particle size. International Journal of Mineral Processing, vol. 42. pp. 169-190. [ Links ]
Senior, G.D. and Thomas, S.A. 2005. Development and implementation of a new flowsheet for the flotation of a low grade nickel ore. International Journal of Mineral Processing, vol. 78. pp. 49-61. [ Links ]
Wiese, J., Harris, P., and Bradshaw, D. 2006. The role of the reagent suite in optimizing pentlandite recoveries from the Merensky reef. Minerals Engineering, vol. 19. pp. 1290-1300. [ Links ]
Wiese, J.G., Harris, P.J., and Bradshaw, D. J. 2008. The use of very low molecular weight polysaccharides as depressant in PGM flotation. Minerals Engineering, vol. 21. pp. 471-482. [ Links ]
Wellham, E.J., Elber, L., and Yan, D. S. 1992. The role of carboxy methyl cellulose in the flotation of a nickel sulfide transition ore. Minerals Engineering, vol. 5. pp. 381-395. [ Links ]
Xu, R.H. 1999. Studies on behavior of serpentines in low-grade Pt-Pd ore of Jinabaoshan by flotation. Master's dissertation, Kunming University of Science and Technology. [ Links ]
Paper received Dec. 2013
Revised paper received Nov. 2014