Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Journal of the Southern African Institute of Mining and Metallurgy
versão On-line ISSN 2411-9717
versão impressa ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 no.6 Johannesburg Jun. 2015
PLATINUM CONFERENCE PAPERS
Fire and brimstone: The roasting of a Merensky PGM concentrate
R.I. RambiyanaI; P. den HoedII; A.M. Garbers-CraigIII
ICentre for Pyrometallurgy, Department of Materials Science and Metallurgical Engineering, University of Pretoria, now working for Anglo American Platinum
IIAnglo American Technical Solutions
IIICentre for Pyrometallurgy, Department of Materials Science and Metallurgical Engineering, University of Pretoria
SYNOPSIS
Four sulphide minerals - pyrite (FeS2), pyrrhotite (Fe1_xS), pentlandite ([Ni,Fej9S8), and chalcopyrite (CuFeS2) - contain the base metals and most of the iron in concentrates of platinum group metals (PGMs). In the pyrometallurgical processing of PGM concentrates these sulphides form a matte during smelting, and iron and sulphur are removed from the matte during the converting process. This paper discusses the roasting of Merensky concentrate in air before smelting, with the purpose of reducing the matte load to the converter.
Roasting tests were conducted in a bench-scale rotary kiln at temperatures from 350°C to 700°C. The concentrate tested contained 17.4% sulphur and consisted of 23% pyrrhotite, 16% pentlandite, 11% chalcopyrite, and 2% pyrite. The particles were fine (d50= 22 μm), and all the sulphide particles were liberated. Roasting in air at 550°C and 650°C for 20 minutes removed respectively 60% and 70% of the sulphur. The iron in the sulphides was oxidized to Fe3O4 (magnetite) at temperatures below 500°C and to Fe2O3 (haematite) at temperatures above 550°C. At 700°C the bed sintered and copper oxides formed. At temperatures below 450°C oxidation was incomplete: pyrrhotite remained and only 30% of the sulphur was removed. Smelting tests were conducted to assess matte fall and the deportment of copper and nickel to matte. It was evident that roasting resulted in lower matte falls (a drop of approximately 60%) compared with matte falls from unroasted concentrate. The iron and sulphur levels in the matte were reduced to below 3.5% and 22% respectively.
This paper also briefly describes the mechanisms by which pyrrhotite, chalcopyrite, and pentlandite are oxidized during roasting. For chalcopyrite, the mechanism proceeds through an intermediate solid solution phase, which extends from Cu1.02Fe1.04S2to Cu2.04Fe0.72S2to a copper-rich solid solution of bornite (Cu4Fe14S4-Cu2S). The oxidation of pentlandite proceeds through a monosulphide solid solution (Ni0.39Fe0.53S-Ni0.74Fe0.15S) to a solid solution of heazlewoodite ([Ni,Fe]3±xS2). These mechanisms are explored in relation to chemical thermodynamics and microstructures.
Keywords: roasting, PGM, concentrate, pyrrhotite, pentlandite, chalcopyrite, smelting, matte, base metal
Introduction
Producers of nickel and copper have been partially roasting concentrates to reduce levels of sulphur and volatile impurities such as arsenic, antimony, and lead for many years (US Environmental Protection Agency, 1995). At Sudbury (Glencore) and Thompson (Vale Inco), concentrate is roasted in fluidized beds (two at each plant) before smelting. The grate diameters of the roasters at Sudbury are 5.6 m, and the freeboard 8 m, while the grate diameters at Thompson are 5.5 m, and the freeboard 6.4 m. The expanded freeboard begins 6.5 m above the grate (Warner et al., 2007). The roasters at Thompson process approximately 50 dry t/h of concentrate. The bed temperatures of the roasters at Thompson and Sudbury are respectively 600°C and 760°C. Both roasters operate under oxidizing atmospheres of air or oxygen-enriched air. Approximately 40% of the sulphur is removed from the concentrate at Thompson, and 70% at Sudbury (Pandher and Utigard, 2010).
Roasting can in principle be applied to PGM concentrates, which besides gangue minerals (mostly minerals in the pyroxene group) comprise pyrite (FeS2), pyrrhotite (Fe1_xS), chalcopyrite (CuFeS2), and pentlandite ([Ni,Fe]9S8). The base metal sulphides are the very same minerals roasted in the copper and nickel industries. In the ConRoast process developed at Mintek, PGM concentrates are dead-roasted to remove all of the sulphur (Jones, 1999). Subsequent smelting under reducing conditions produces a Cu-Ni-bearing alloy, which collects the PGMs. Many of the aims of roasting could be met by partially oxidizing the base metal sulphides. Partial roasting would desulphurize the concentrate, oxidize some or most of the iron, and the roasted concentrate would still produce a matte.
This study focused on partial roasting of a Merensky concentrate. It examined how the conditions of roasting affect the degree of desulphurization, which phases form, how these phases affect matte fall, and the deportment of base metals to the matte. Mechanisms for the oxidation of pyrrhotite, pentlandite, and chalcopyrite were also determined.
Experimental
Sample
The concentrate sample - a product of flotation - originated from the Merensky Reef in Limpopo Province, South Africa. The bulk modal analysis of the concentrate, as determined with a Mineral Liberation Analyser (MLA), is given in Table I. The main sulphide minerals present in the concentrate were pyrrhotite (Fe1_xS), pentlandite ([Ni,Fe]9S8), and chalcopyrite (CuFeS2); together accounting for 50.1% of the mass of the concentrate. The metal sulphides in sub-samples showed a degree of variation with respect to phase compositions (Rambiyana, 2015). Attaching a fixed set of conditons to a degree of oxidation is therefore problematic.
Procedure
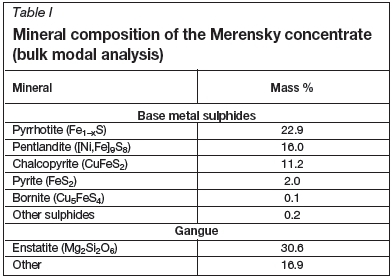
Roasting tests were conducted in a rotating-tube furnace (Figure 1). This reactor provided good gas-solid mixing and contact for the duration of a test and readily dissipated heat from the exothermic reactions. The furnace was fitted with a quartz work tube, 100 mm in internal diameter and 1150 mm long. The work tube was externally heated, which resulted in a hot zone of 550 mm. The tube was fitted with lifters to ensure good gas-solid mixing and contact. The roasting tests were conducted under isothermal conditions at temperatures ranging from 400°C to 650°C and a residence time of 20 minutes. The work tube was purged with air at a flow rate of 42 NL/min. Roasting was conducted with the reactor run in continuous mode.
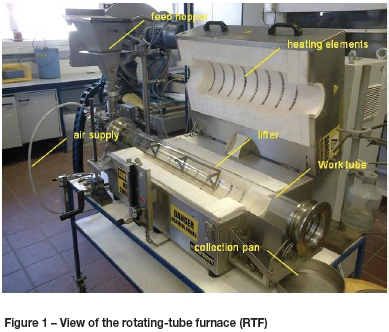
The sulphation reactions were tested in an angular reciprocating capsule that was heated in a horizontal split-shell furnace. This capsule rotated about its longitudinal axis, but alternated between clockwise and anticlockwise (Figure 2). The capsule had an internal diameter of 44 mm, a working zone of 120 mm, and a volume of 954 cm3. It was also fitted with lifters. The temperature of the bed was measured with a K-type thermocouple. Tests were carried out on 50 g samples held at 500°C under controlled atmospheres (air at 1 and 2 bar) for one hour. The pressure in the capsule was recorded for the duration of the tests. The capsule provided 0.0148 moles of oxygen for every 1 bar of pressure. Smelting tests were conducted in a vertical-tube furnace. The mullite work tube had an internal diameter of 80 mm and a hot zone of 150 mm. Unroasted concentrate, concentrate roasted at 550°C, and concentrate roasted at 650°C were smelted in alumina crucibles. A flux consisting of 10 g Al2O3 and 10 g CaO for every 100 g of concentrate was added to the charge. The charge was mixed well and smelted at 1500°C for 30 minutes. The sample was furnace-cooled.
Analytical techniques
The products of roasting and smelting were examined and analysed by an array of techniques. These included X-ray diffraction analysis (XRD); scanning electron microscopy (SEM), using energy dispersive spectrometry (EDS); quantitative evaluation of minerals by scanning electron microscopy (QEMSCAN); and quantification of the amount of ferromagnetic material. XRD analysis was performed with a PANalytical X'Pert Pro powder diffractometer in θ-θ configuration with an X'Celerator detector and variable divergence and receiving slits. The radiation was Fe-filtered Co-Ka (λ=1.789 Å). Phases were identified by means of X'Pert Highscore Plus software. SEM-EDS was performed with a Mineral Liberation Analyser to identify phases and analyse their compositions. QEMSCAN-EDS was used to create mineral maps of the samples. The percentage ferromagnetic material was determined with a Satmagan 135 (Rapiscan Systems). This instrument measures the ferromagnetic signal from a sample and correlates it with magnetite content.
Results
Roasting mechanisms
Roasting tests focused on two temperatures, 450°C and 650°C. These temperatures were chosen based on data published in the literature for the pertinent systems Fe-Ni-S and Fe-Cu-S. Rates of oxidation are too slow at temperatures below 400°C, while particles sinter and stick to the wall of the work tube at temperatures above 700°C. Tests were run in air at temperature for residence times ranging from several minutes to 25 minutes. Most tests were run with the sample passing through the kiln in 20 minutes. The phase chemistry of the roasted samples was analysed using SEM-EDS and XRD. By combining the conditions of roasting and phase chemical results with phase diagrams and thermo-chemical data, the mechanisms for the oxidation of the three primary sulphide minerals in the PGM concentrate were postulated.
Pyrrhotite (Fe1-xS)
Pyrrhotite reacted with oxygen to form iron oxide. This reaction was rapid compared with the oxidation of the base metal sulphides. In the 400-650°C temperature range pyrrhotite disappeared within 20 minutes. Chemical thermodynamics predicts that oxidation should result in the formation of Fe2O3 (haematite) (Muan and Osborn, 1965: Figure 12). However, Fe3O4 (magnetite) formed as an intermediate phase. These observations are consistent with the assumption that in the temperature range 400-650°C, the oxidation of Fe3O4 to Fe2O3 is relatively slow and that the oxidation rate increases with temperature. At temperatures below 500°C oxidation did not proceed much beyond Fe3O4. As temperatures increased from 500°C to 650°C, increasing amounts of Fe2O3 formed and less Fe3O4. The reaction occurred at the interface between pyrrhotite (Fe1-xS) and the iron oxide. The reaction might proceed by means of a shrinking core but, given that the pyrrhotite is porous, it might occur at several loci within each particle.
As the conversion of Fe3O4to Fe2O3also occurred during the oxidation of the base metal sulphides, it is discussed in more detail in a subsequent section of this paper. This 'duplex layer' has been observed in other studies on the roasting of base metal sulphides (Xia, Pring, and Brugger, 2012).
Chalcopyrite (CuFeS2)
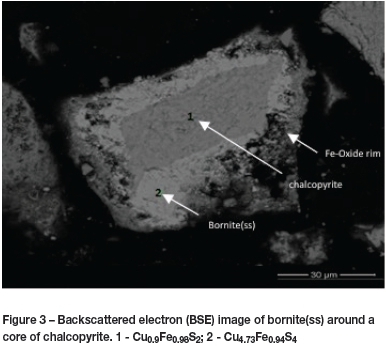
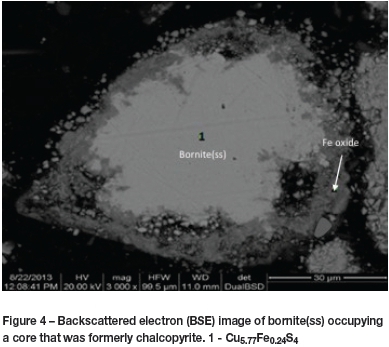
Chalcopyrite particles in the initial stages of oxidation had compositions at the iron-rich end of the bornite solid-solution series. Chalcopyrite occupied a core that shrunk as bornite(ss) developed around it (Figure 3). Iron oxide (Fe2O3 or Fe3O4) formed on the surface of the bornite(ss). The disappearance of chalcopyrite was rapid. Further oxidation proceeded by the reaction of oxygen with iron and sulphur in bornite(ss), which retained its structure but became increasingly depleted in iron and (somewhat less) in sulphur (Figure 4). It was difficult to accurately determine the change in bornite(ss) phase compositions radially with SEM-EDS, as the particles were too fine. It is therefore not clear whether the chalcopyrite disappeared before bornite(ss) started to oxidize. Cu-rich end-members of the bornite(ss) were not detected with chalcopyrite cores in a particle. Reactions were faster at higher temperatures. Chalcopyrite disappeared after roasting for 20 minutes at 450°C and 650°C. However, the bornite(ss) was richer in copper at higher temperatures and richer in iron at lower temperatures after 20 minutes. This empirical evidence is in disagreement with the phase relations in the system Fe-Cu-S (Rambiyana, 2015). An intermediate solid solution (iss) was found to be stable at temperatures above 400°C, occupying a region of the phase diagram between CuFeS2 (chalcopyrite) and the bornite(ss). The presence of iss could not be confirmed in this study.
Pentlandite ([Ni,Fe]9S8)
An initial rapid reaction resulted in pentlandite losing iron through oxidation to Fe3O4 and transforming into the mss (monosulphide solid solution) phase, which became progressively depleted in iron through further oxidation. An individual particle could exhibit variable compositions of the mss phase (Figure 5). More Fe3O4 (magnetite) formed and was oxidized to Fe2O3. The Ni-rich mss finally became unstable and underwent a structural change to heazle-woodite (ss). This agrees with the work of Zamalloa and

Utigard (1996), who identified (Ni,Fe)3±xS2 (heazlewoodite) in particles roasted at 747°C. This reaction sequence was observed at all temperatures above 400°C. Pentlandite disappeared after 10 minutes at all temperatures above 400°C. Heazlewoodite (ss) appeared after 20 minutes at temperatures above 650°C.
The absence of sulphates
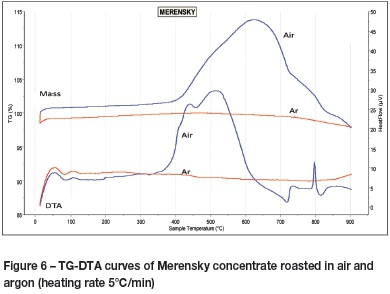
The formation of sulphates during roasting is undesirable as sulphates retain sulphur. Sulphate formation can, however, be avoided by considering the thermodynamic relations depicted in the predominance diagrams of Fe-Ni-S-O and Fe-Cu-S-O. High partial pressures of SO2and O2tend to stabilize the metal sulphates FeSO4 and Fe2(SO4)3. To avoid the formation of sulphates during roasting of base metal sulphides, pSO2 should therefore be kept low (<1%). However, higher partial pressures of SO2 may be established inadvertently _ fixed beds of fine particles promote high SO2 partial pressures within the bed, as SO2 is unable to diffuse rapidly to the free surface of the bed. Examples include particulate beds contained in crucibles or boats used in thermogravimetry and in work utilizing muffle furnaces. Disturbing the bed frees the 'trapped' SO2. This was accomplished in the rotating-tube furnace by the action of the lifters in the work tube. Compressed air was also forced in at the feed end of the work tube and extracted by means of an extraction duct inserted at the discharge end. A high pO2 and a low pSO2 were therefore maintained in the tube. No sulphates were detected in any of the products from tests conducted in the rotating-tube furnace. Roasting of concentrate in a thermobalance and the angular reciprocating capsule, however, produced metal sulphates, as indicated by a gain in mass shown by the thermobalance (Figure 6). The sample started to gain mass from approximately 350°C, and the gain became significant between 400°C and 620°C (Figure 6). It is at these temperatures, under high SO2 partial pressures, that metal sulphates can be expected to form. At temperatures greater than 620°C the sample lost mass. This loss is associated with the decomposition of sulphates, which are thermodynamically unstable at high temperatures. The temperatures for the thermal decomposition of ferrous sulphate, copper sulphate, and nickel sulphate are reported in the literature (Kolta and Askar, 1975). The two peaks in the TG curve - one at 725°C, the other at 830°C - indicate the decomposition of different sulphates at their respective thermal stability limits.
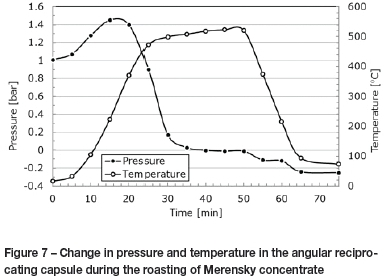
Sulphates were also formed in the angular reciprocating capsule, as high SO2partial pressures were established by maintaining a closed system. The pressure in the capsule increased while the capsule was being heated to temperature (Figure 7). The initial increase in pressure can be attributed principally to the thermal expansion of gas (air) in the freeboard. The pressure dropped when the temperature exceeded approximately 200°C, and the final recorded pressure was lower than the pressure in the system at the beginning of the test. From these measurements, and from an understanding of the conditions that promote sulphate formation, it can be concluded that oxygen was withdrawn from the gas phase and reacted with the sulphides to form sulphates.
Sulphur removal and the formation of iron oxides
The primary objective of roasting is to lower the levels of sulphur in a concentrate. A second objective is the preferential oxidation of iron in the base metal sulphides so that the iron can be removed in the slag phase during smelting. Iron is oxidized to either Fe2O3 or Fe3O4. Where possible, magnetite formation should be avoided as iron-bearing spinels form a viscous intermediate layer between the matte and slag layers during smelting. This viscous layer results in increased levels of matte entrainment in, and PGM losses to, the slag. The build-up of Fe3O4or other spinels during smelting also reduces furnace capacity (Jones, 1999).
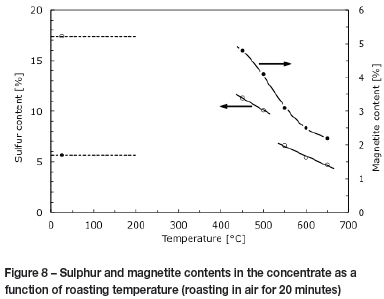
The amount of either Fe3O4 or Fe2O3 in the product is also linked to temperature (Muan and Osborn, 1965). The unroasted concentrate contained 17.4% sulphur. The degree of desulphurization increases with increasing temperature, as reactions are faster at higher temperatures and, for the same duration of oxidation (20 minutes), the extent of desulphurization is higher. Since the concentrate sample consisted of different proportions of pyrrhotite, pentlandite, and chalcopyrite, the overall degree of desulphurization reflects the joint extents of oxidation of these different sulphide phases. At 550°C the sulphur content in the roasted concentrate dropped to 6.5% (a decrease in sulphur content of approximately 60%), while at 650°C it dropped to just over 5% (a decrease of 70%).
The magnetite content also decreased with temperature (Figure 8). Oxidation produced both Fe2O3 and Fe3O4. At approximately 400°C, after 20 minute of roasting, the magnetite content was at its highest and decreased steadily with increasing temperature. Thermodynamics predicts that haematite is the stable form of iron oxide in air, in the temperature range used in this study (Muan and Osborn, 1965. The presence of magnetite therefore reflects non-equilibrium conditions.
Smelting
When concentrate is smelted, PGMs are collected in the matte phase. The principal aim of partially roasting the concentrate before smelting is to decrease the matte fall, thereby resulting in a higher PGM concentration in the matte phase. It is thus important to know how a roasted concentrate will behave on smelting. Bench-scale smelting tests were therefore conducted on the roasted concentrate, using alumina crucibles. The smelting tests were evaluated according to the matte fall (as a percentage of material charged to the crucible), the deportment of Ni and Cu to the matte, the collection of PGMs in the matte (if this could be measured accurately on a small scale), as well as the composition of the matte.
Since roasting lowered the sulphur concentrations in the concentrate by approximately 60%, from 17.4 % to 6.5 % at 550°C, while the concentration of magnetite formed decreased from approximately 4.8% to 3.1% as the temperature increased from 450°C to 550°C, it was decided to do the smelting tests only on the roasted concentrate samples of lower magnetite content, i.e. the samples that were roasted at 550°C and 650°C. Matte was collected from the smelting of fresh concentrate, while matte associated with an alloy phase was collected from smelting of concentrate roasted at 550°C (Figure 9). Smelting of the concentrate roasted at 650°C produced only an alloy. In all three tests the matte or alloy collected in fine beads and prills, which did not fall under gravity to the bottom of the crucible. The viscosity of the slag was therefore too high to facilitate the coalescence of matte or alloy into a button within 30 minutes. Dispersed matte hindered the direct measurement of matte fall. Matte fall was subsequently estimated at 45% for the unroasted concentrate and 15% for the concentrate roasted at 550°C, using a least-squares regression of concentrate and matte compositions within the framework of a mass balance. These values are lower than the matte falls calculated from all sulphides that can possibly report to the matte: 52% for the unroasted concentrate, 21% for the concentrate roasted at 550°C, and 17.6% for the concentrate roasted at 650°C.
Although matte (from the unroasted and 550°C roasted concentrates) and alloy (from the 650°C roasted concentrate) did not coalesce, the compositions of matte and alloy beads and prills as well as the slags could be determined. The matte from the concentrate roasted at 550°C comprised Ni3S2 (heazlewoodite), Cu9S5 (digenite), Cu1.97S (djurleite), and Cu2S (chalcocite) (Table II). The PGM-containing alloy was associated only with nickel-copper-iron alloys (Figure 9C). Some nickel was lost to the slag (estimated in a mass balance calculation to be less than 10% in silicates and spinel). Copper was not detected in any of the phases in the slag.
The formation of a Cu-rich alloy in the smelted product of the concentrate roasted at 650°C was unexpected (Figure 10). With 5% residual sulphur in this roasted product, up to 10% matte was expected to form. The alloy was entrained in an iron-rich spinel in a silicate matrix of variable composition (Table III).
Conclusions
➤ It is possible to selectively oxidize the iron in sulphides that are present in a Merensky concentrate, leave the nickel and copper in sulphide phases, and produce a matte on smelting. This was achieved by roasting the concentrate at 550°C for 20 minutes
➤ The oxidation of the iron-containing sulphide phases at temperatures between 500°C and 650°C proceeded as follows:
- Pyrrhotite oxidized to magnetite, which in turn oxidized to haematite. The extent of haematite formation increased with roasting temperature
- Chalcopyrite oxidized to form a bornite(ss) phase, with compositions close to the Fe-rich end member at 550°C and a Cu-rich end member at 650°C
- Pentlandite oxidized to monosulphide solid solution (mss). The mss composition tended towards the NiS (millerite) end-member of the solution with oxidation at higher temperatures.
➤ Roasting at 550°C lowered the sulphur levels by at least 60%, from 17.4% to 6.5% total sulphur, and at 650°C, sulphur was lowered by 70%, from 17.4% to 4.9% total sulphur
➤ The magnetite content of the calcine roasted at 550°C was double that of unroasted concentrate
➤ As long as the oxygen partial pressure in the rotary furnace remained high (close to that of air), metal sulphates did not form
➤ Smelting tests revealed that concentrates roasted at 550°C for 20 minutes produced matte and a greatly reduced matte fall. The iron levels in the matte were below 3.5%. The sulphur content of this matte was just above 20%, compared to a sulphur content of more than 30% for matte from unroasted concentrate
➤ The matte produced from roasted concentrate resembled the converter matte in composition.
Acknowledgments
The authors would like to thank Lonmin for supplying the concentrate, and gratefully acknowledge the technical support of Dr Lloyd Nelson and Rodney Hundermark from Anglo American Platinum, as well as Rian Bezuidenhout and Burger van Beek from Lonmin. A special word of thanks goes to our colleagues in the Department of Material Science and Metallurgical Engineering at the University of Pretoria. At Anglo American our thanks go to Dr L.J. Bryson (Head of Hydrometallurgy) and Dr R.P. Schouwstra (Head of Mineral and Process Research) for the use of the facilities at Technical Solutions as well as their technical support.
This work is based on the research supported in part by the National Research Foundation of South Africa (Grant number TP1208219517).
References
Geldart, D. 1973. Types of gas fluidization. Powder Technology, vol. 7. pp. 185-195. [ Links ]
Jones, R.T. 1999. Platinum smelting in South Africa. South African Journal of Science. vol. 95, no. 11-12. pp. 525-534. [ Links ]
Knowlton, T.M. 2002. A review of catalytic fluidized-bed reactors in the chemical and petrochemical industries. IFSA 2002, Industrial Fluidization South Africa. Luckos, A. and Den Hoed, P. (eds). Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 3-31. [ Links ]
Kolta, G.A. and Askar, M.H. 1975. Thermal decomposition of some metal sulphates. Thermochimica Acta, vol. 11, no. 1. pp. 65-72. [ Links ]
Muan, A. and Osborn, E.F. 1965. Phase Equilibria among Oxides in Steelmaking. Addison-Wesley, Reading, MA. [ Links ]
Pandher, R. and Utigard, T. 2010. Roasting of nickel concentrates. Metallurgical and Matererials Transaction B, vol. 41, no. 4. pp. 780-789. [ Links ]
Rambiyana, R.I. 2015. Partial roasting of a PGM concentrate. MEng dissertation, University of Pretoria, South Africa. [ Links ]
US Environmental Protection Agency. 1995. Compilation of Air Pollutant Emission Factors. Volume 1: Stationary Point and Area Sources. Chapter 12. Office of Air Quality Planning and Standards (OAQPS) and Office of Air and Radiation (OAR) Research Triangle Park, NC. [ Links ]
Warner, A.E.M., Diaz, C.M., Dalvi, A.D., Mackey, P.J., Tarasov, A.V., and Jones, R.T. 2007. JOM World Nonferrous Smelter Survey. Part IV: Nickel Sulphide. JOM, vol. 59, no. 4. pp. 58-72. [ Links ]
Xia, F., Pring, A., and Brugger, J. 2012. Understanding the mechanism and kinetics of pentlandite oxidation in extractive pyrometallurgy of nickel. Minerals Engineering, vol. 27-28. pp. 11-19. [ Links ]
Zamalloa, M. and Utigard, T.A. 1996. The behaviour of Ni-Cu concentrate in an industrial fluid bed roaster. Canadian Metallurgical Quarterly, vol. 35, no. 5. pp. 435-449. [ Links ]














