Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.6 Johannesburg Jun. 2015
PLATINUM CONFERENCE PAPERS
Corrosion resistance of laser-cladded 304L stainless steel enriched with ruthenium additions exposed to sulphuric acid and sodium chloride media
J. van der MerweI, II; D. TharandtI, II, III
ISchool of Chemical and Metallurgical Engineering, University of the Witwatersrand, Johannesburg, South Africa
IIDST/NRF Centre of Excellence for Strong Materials, University of the Witwatersrand, Johannesburg, South Africa
IIIWorley Parsons, Johannesburg
SYNOPSIS
The corrosion behaviour of 304L stainless steel laser-cladded with various amounts of ruthenium (Ru) was evaluated in solutions of sulphuric acid and sulphuric acid plus sodium chloride at 25°C and 45°C by open-circuit potential and cyclic potentiodynamic polarization tests. In general, the addition of Ru to the stainless steel increased its corrosion resistance in 1 M H2SO4, as well as in 1 M H2SO4 plus 1 wt% NaCl. This was observed for a number of parameters such as corrosion rate, corrosion potential, open-circuit potential, and current density. However, increasing the amount of Ru added beyond a certain level did not result in further improvement in corrosion protection. For each environment there is an optimal Ru concentration for the best corrosion protection. For example, in 1 M H2SO4 at 25°C, 2.44 wt% Ru shows the least active surface in terms of corrosion. Further research into ruthenium coatings on stainless steels is recommended.
Keywords: corrosion protection, ruthenium, laser cladding, 304 stainless steel
Introduction
Corrosion is responsible for significant economic loss in all types of industries due to equipment failures and additional maintenance requirements. It can be prevented or reduced by applying surface coatings to the metal. The evaluation of a more corrosion-resistant surface coating on 304L stainless steel is the subject of this investigation.
It is well known that the corrosion resistance (electrochemical and pitting corrosion) of all types of stainless steels is significantly increased by alloying it with small amounts of platinum group metals (PGMs) (Sherif et al., 2009). Ru is by far the least expensive metal of the PGM family and therefore is most applicable in industry for the passivation of stainless steels. It has been observed that the addition of small amounts of Ru improves the corrosion resistance of stainless steel (Potgieter, Ellis, and van Bennekom, 1995). Bulk alloying is still considered expensive, and since corrosion is a surface phenomenon, recent research (Lekala, van der Merwe, and Pityana, 2012) indicates a tendency to add the alloy only to the surface, where corrosion protection is most required.
It is now generally known (Potgieter, Ellis, and van Bennekom, 1995) that during active corrosion, ruthenium additions increase the resistance of stainless steel to anodic dissolution and lower the hydrogen over-potential. This implies that ruthenium inhibits the corrosion of the alloy by a combination of these two mechanisms. During active dissolution, ruthenium increases the corrosion potential and lowers the critical as well as the passivation current density. Potgieter, Ellis, and van Bennekom (1995) showed that stainless steel alloyed with minor ruthenium additions passivates spontaneously due to the formation of a stable passive surface layer with a significantly increased corrosion resistance. This shifts the corrosion potential of these alloys towards more noble (more positive) values. The mechanism of corrosion also depends on the medium of exposure.
Experimental procedure
Test materials
Stainless steel 304L base-plate 5 mm in thickness was used as the substrate for all the test samples. A mixture of 304L stainless steel powder and ruthenium powder was used to clad the base-plate using a laser surface-cladding technique. The ruthenium powder was added to the stainless steel powder in varying ratios to obtain target Ru contents in the coating of 1 wt%, 2 wt%, 3 wt%, 4 wt%, and 5 wt%.
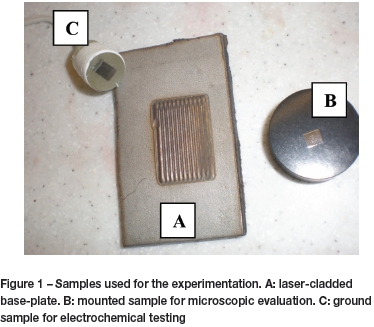
The plates were plasma-cut into approximately 40 x 60 mm sections and cleaned with acetone before cladding. The cladded portion was 20 x 30 mm, providing approximately 600 mm2 of cladding. Figure 1A shows the stainless steel after laser cladding, from which the samples were cut.
Laser surface alloying technique
The laser cladding was performed using a 4.4 kW Rofin Sinar diode-pumped Nd:Y AG laser. The 1.064 μm radiation was delivered via a 400 μm core diameter step index optical fibre to a 200 mm focal length collimator. The collimated beam was focused with a 300 mm focal length lens. The optical assembly was mounted on a KIKA KR60L30HA sixaxis articulated arm robot to control the welding process. The laser spot size was 2 mm in diameter. The stepover for all the samples, i.e. the centre-to-centre distance of successive weld beads, was 0.8 mm. The laser power used was 1200 W and the scan speed was 2 m/min bi-directional. The carrier shield gas was argon at a flow rate of 3 standard L/min.
The cladded plate was cut into a number of approximately 5 x 5 mm samples for assessment of the alloyed surface and the cross-sectional microstructure, as well as electrochemical tests.
Scanning electron microscopy (SEM)
The samples for SEM tests were mounted separately in Bakelite® powder using a mounting press. Two samples for each composition were mounted - one such that the alloyed surface could be examined, and the second sample such that the cross-section of the weld could be examined. The samples were ground in stages to 1200 grit size using silicon carbide paper, as were the samples for electrochemical testing. The samples were then polished using 3 μm diamond powder on an automated polishing machine. The samples were cleaned with ethanol and dried with compressed air. The clean and dry samples were then electrolytically etched in 10 wt% oxalic acid solution for 30 seconds. The microstructures were evaluated using a Zeiss Axiotech 25 HD microscope. Figure 1B shows a mounted sample used for microscopic evaluation.
SEM is a semi-quantitative method of chemical analysis that provides only an indication of composition. The chemical composition of the alloyed surface was measured by scanning its surface area as well as the cross-sectional area of the sample using the energy dispersive spectroscopy (EDS) capability of the Zeiss Sigma field emission SEM. EDS was conducted at a working distance of approximately 8.5 mm and an acceleration voltage of 20.0 kV. The overall composition was determined by averaging the measured compositions.
Electrochemical tests
The samples prepared for electrochemical tests were cold-mounted in epoxy resin such that the alloyed surface would be exposed to the corrosive environment. An example of a 1200 grit ground sample, ready for testing, is shown in Figure 1C.
The electrochemical tests were conducted in an electrochemical cell consisting of the working electrode (the cold-mounted sample), a platinum counter-electrode, and a silver/silver chloride reference electrode. The electrochemical polarization measurements were carried out by an autolab potentiostat. Nova software was used to simulate the test procedures as well as to analyse the resultant potentio-dynamic polarization curves.
The potentiodynamic polarization procedure consisted of the following consecutive steps:
➤ Open-circuit potential (E vs time) for 12 hours
➤ Anodic scan from -500 mV to +1100 mV at a scan rate of 1 mV/s
➤ Polarization at -500 mV for 5 minutes
➤ Anodic scan from -500 mV to +1100 mV at a scan rate of 1 mV/s.
The tests were conducted according to the ASTM G5 standard. The corrosive environment for each sample was altered by varying the medium and the temperature. The two solutions used were 1 M sulphuric acid and 1 M sulphuric acid with 1 wt% sodium chloride. The samples were exposed at temperatures of 25°C and 45°C; the temperatures were kept constant by a thermostat-controlled water bath.
Results and analysis
Energy dispersive spectroscopy
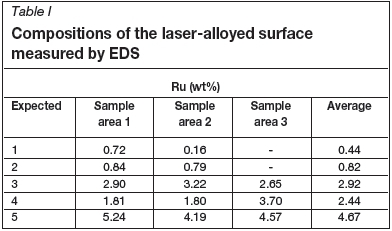
The compositions of the laser cladded surfaces, as obtained by EDS, are shown in Table I.
The stainless steel base-plate was also analysed. The composition is shown in Table II.
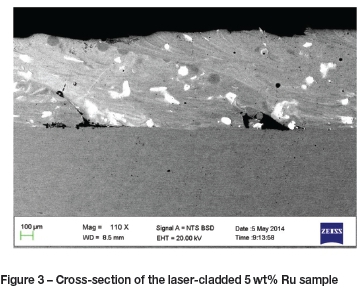
The EDS results show that the expected Ru compositions were not attained on all samples, and the composition varied significantly between different areas on the same sample. This is a result of the actual cladding procedure, since the variation of the base-plate composition was negligible. On some samples, ruthenium-rich stringers were observed where the ruthenium was well mixed with the stainless steel powder; in those areas the Ru concentration was very close to that expected. In other areas Ru islands were observed where the Ru concentration was up to 100 wt%. A consistent coating thickness was obtained, but the coating was not homogeneous at higher Ru concentrations and could thus not produce a consistent protective layer. Figures 2 and 3 show examples of the images obtained and analysed; the white spots represent pure Ru. The average compositions of these cladded samples are given in Table III.
Electrochemical testing
A number of parameters were used to indicate corrosion resistance. These included the corrosion rate (mm/a); the observed corrosion potential, Ecorr (V); the passivation exchange current density, ipass (A/cm2); the open-circuit potential, OCP (V); and the critical exchange current, icrit (A).
1 M H2SO4 solution at 25°C
Increasing the Ru concentration within the cladded layer was expected to improve corrosion resistance. From the log i vs E curve (Figure 4) it is evident that small additions of Ru to the stainless steel surface protect in 1 M H2SO4 solution. The passivation current density for the samples containing Ru is orders of magnitude smaller than when no Ru is added. However, passivation does not occur over a large potential range at a specific current density normally associated with a stable protective film on the surface. For samples with Ru cladding, the current density increases in this passivation region as the potential increases until the transpassive potential is reached. The expected step increases with increasing Ru concentration are not evident. Averaging all the results and looking at a combination of all the above-mentioned parameters for corrosion protection, a very clear ranking order is observed. The order of decreasing corrosion resistance in 1 M H2SO4 solution at ambient temperatures is 2.44 wt%, 0.82 wt%, 2.92 wt%, 4.67 wt%, 0.44 wt%, stainless steel blank, and 0 wt% Ru.
The stainless steel blank sample (no cladding) shows a typical curve with distinct active, passive, and transpassive regions. The cladded sample with no Ru added indicates very low corrosion resistance in this environment, with a more active corrosion potential indicating that a welded region would be more susceptible to corrosion, as expected. At 0.44 wt% Ru, the benefit of corrosion protection can be seen, and at higher Ru concentrations more noble corrosion potentials are seen, indicating the formation of a more stable passive layer.
Samples that exhibited high corrosion potentials had a lesser tendency to corrode since a high potential energy is required to break down or corrode the alloy. High positive potentials are an indication of spontaneous passivation of the surface of the sample (Papavinasam, 2013). From the trend shown in Table IV, it is clear that the maximum corrosion protection is attained at 2.44 wt% Ru, closely followed by 0.82 wt% Ru (both of which indicated spontaneous passivation), then 4.67 wt% Ru, 2.92 wt% Ru, 0.44 wt% Ru, no Ru, and finally the stainless steel blank.
Figure 5 shows that a stable potential was achieved over a short period of time; most notably for the 2.44 wt% Ru sample. The attained potentials with Ru addition were in the noble po-tential region. Higher, positive OCPs were obtained with increasing Ru, peaking at 2.92 wt% Ru, followed by 0.82 wt%, 4.67 wt%, 0.44 wt%, and 2.44 wt% Ru.
1 M H2SO4 + 1 wt% NaCl solution at 25°C
Addition of sodium chloride to the sulphuric acid solution increased the corrosivity of the environment due to the chloride ions attacking the passive layer formed on the surface of the cladded material. A clearer relationship between increasing Ru concentration and improved corrosion protection became evident. Figure 6 shows that the corrosion potential increased with increasing Ru content. The presence of chloride ions in the solution accelerated the damage to the passive layer.
The effects of variations in Ru concentrations in the samples were also observed in the tests with the acid and salt solution. Table V shows a slightly different trend with regard to specific corrosion protection values, but clearly indicates that cladding with stainless steel alone, without any Ru, decreases corrosion resistance, while increasing the amount of Ru added improves the corrosion protection significantly in 1 M H2SO4 with 1 wt% NaCl. Samples with 0.44 and 2.44 wt% Ru addition were damaged during the experimental stage and no further results could be obtained from these.
The attained open-circuit potentials were negative only for the cladding without Ru and the stainless steel blank samples, while the addition of Ru brought them into the noble region The clear trend is, in order of increasing corrosion protection: 0 wt% Ru, stainless steel blank, 0.82 wt% Ru, 2.92 wt% Ru, and 4.67 wt% Ru.
1 M H2SO4 + 1 wt% NaCl solution at 45°C
At higher temperature (45°C) in an acid environment with the presence of chloride ions there was no significant improvement in corrosion resistance with increasing Ru content. The stainless steel blank as well as the 0 wt% Ru sample behaved similarly, and all samples containing Ru behaved very similarly, as can be seen from Figures 7 and 8. A notable improvement in corrosion protection is observed with the addition of 0.82% Ru, but no additional benefit was observed at higher Ru contents.
Discussion
The laser cladding method appears to have resulted in lower Ru compositions in the cladding than in the powder used. Although a uniform coating depth of almost 1 mm was obtained, Ru distribution was not uniform. The high variability of the Ru content of the cladding is the most likely cause of the variability of the electrochemical results, since each fresh surface exposed had a slightly different composition and structure. Repeatable results were obtained with the stainless steel blank sample, but the cladding introduced significant variability. As can be seen from Figures 2 and 3 as well as Table I, the Ru concentration is highly variable between specific small areas within the plate. It should be noted that the EDS results are only for comparing the various samples - EDS is by no means the most accurate method for determining chemical composition.
The polarization curves clearly indicate an improved corrosion resistance with the addition of Ru in 1 M H2SO4 and 1 M H2SO4 plus 1 wt% NaCl, especially at higher temperatures. The reason for this is as follows. Since the over-potential of the standard hydrogen reduction reaction is low, the addition of Ru shifts the equilibrium reaction to more active potentials, and this shift is sufficient in a reducing medium to move the system into the passive region and thus reduce the actual corrosion rate of the sample. McGill (1990) also observed this initial rapid corrosion of stainless steel followed by passivation in non-oxidizing acid media such as sulphuric acid (H2SO4). The solution concentration is critical, as too high a concentration results in no passivation since the dissolution rate is too fast (McGill, 1990). Potgieter and Brookes (1995) observed that adding too small an amount of Ru can increase corrosion rates as it increases the efficiency of the cathodic hydrogen reaction. Therefore, passivation is induced only if the passivation potential of the PGM-metal alloy is less than the overpotential of the hydrogen evolution reaction on the alloying PGM. Potgieter and Brookes (1995) conclude that there is a maximum amount of ruthenium that can be added to stainless steel to increase corrosion resistance, beyond which no further improvement is gained. The actual amount depends on the exposure medium and temperature. This is corroborated by the results of the current investigation.
The OCP was expected to increase with time as the formation of a passive region occurs by the dissolution of oxides that accumulate into a protective layer. Ruthenium is expected to become concentrated on the surface as the other components oxidize, thereby stabilizing the protective layer by preventing it breaking down. It was thus expected that increasing the Ru content would increase that stabilizing effect, consequently increasing the resistance of the material to corrosion. OCP values of the claddings containing Ru were in the positive region, but did not simply increase with increasing Ru content. There is a definite optimal Ru concentration for a particular environment.
It is important in the development of any new product or alloy to ensure that it is cost-effective, or there will be only very limited scope for application. In this case, the various compositions of ruthenium in the stainless steel need to be evaluated for cost-effectiveness and compared to other existing materials offering similar levels of corrosion protection. The cost of only the ruthenium metal was taken into account for this evaluation. A 5 wt% Ru coating is required, and it could only be 100-200 μm thick if it were to replace a 316 stainless steel or SAF2205. Such thickness is difficult to achieve at this stage with the laser cladding process. If a 3 wt% Ru coating was sufficient, it could be up to 500 μm thick to compete with SAF2205 on the material costs only. This thickness can be achieved more easily with the laser cladding. A different cladding technique needs to be investigated in order to reduce the thickness of the applied ruthenium alloy. Hastelloy C276, on the other hand, is so expensive that almost any quantity of ruthenium alloyed with 304L stainless steel would be cost-effective providing that it can demonstrate equivalent corrosion protection. Therefore, especially for smaller components, this technology could be very beneficial and improve the lifespan considerably.
Conclusions
➤ The laser surface cladding method was successfully used to add small amounts of ruthenium to a stainless steel cladding in a non-porous and well-adhered layer of uniform thickness. The variability in terms of chemical composition and ruthenium distribution in the cladding need to be improved in future studies.
➤ The addition of Ru to the stainless steel increased its corrosion resistance in 1 M H2SO4, as well as in 1 M H2SO4 plus 1 wt% NaCl. This was observed for a number of variables such as corrosion rate, corrosion potential, OCP, and current density.
➤ Corrosion protection did not improve with increasing additional of Ru beyond a certain level. There is an optimal Ru concentration to be added for a specific environment. By averaging the results a clear ranking order is obtained: for the 1 M H2SO4 solution at 25°C the order of increasing corrosion protection is 0 wt%, 0.44 wt%, 2.92 wt%, 4.67 wt%, 0.82 wt% and best at 2.44 wt%. The addition of 1 wt%NaCl changes that to 0 wt%, 0.82 wt%, 2.92 wt% and 4.67 wt%Ru.
➤ Chloride ions attack the passive layer formed on stainless steel, increasing the corrosion rate. This is also applicable to the Ru-cladded samples, even though corrosion protection is substantially improved.
➤ Increasing the temperature of the 1 M H2SO4 plus 1 wt% NaCl environment to which the samples were exposed increased the corrosivity. Test results showed that the passive layers formed were stable as the temperature increased. A notable improvement in corrosion protection is observed with the addition of Ru under these conditions, but adding Ru above a certain level does not result in any additional benefit for the Ru range observed.
➤ Ruthenium-cladded samples behaved differently in different environments, and hence their application should be carefully selected and evaluated against available types of stainless steel.
References
Lekala, M.B., van der Merwe, J.W., and Pityana, S.L. 2012. Laser surface alloying of 316L stainless steel with Ru and Ni mixtures. International Journal of Corrosion, vol. 2012. pp. 1-4. [ Links ]
McGill, I.R. 1990. Platinum metals in stainless steels - a review of corrosion and mechanical properties. Platinum Metals Review, vol. 34. pp. 85-97. [ Links ]
Papavinasam, S. 2013. Corrosion Control in the Oil and Gas Industry. Elsevier, Amsterdam. Chapter 2. pp. 49. [ Links ]
Potgieter, J.H. and Brookes, H.C. 1995. Corrosion behavior of a high-chromium duplex stainless steel with minor additions of ruthenium in sulfuric acid. Corrosion Engineering, vol. 51, no.4. pp. 312-320. [ Links ]
Potgieter, J.H., Ellis, P., and van Bennekom, A. 1995. Investigation of the active dissolution behaviour of a 22wt%> chromium duplex stainless steel with small ruthenium additions in sulphuric acid. ISIJ International, vol. 35. pp. 197-202. [ Links ]
Sherif, El-Sayed M., Potgieter, J.H., Comins, J.D., Cornish L.A., Olubambi P.A., and Machio C.N. 2009.Effects of minor additions of ruthenium on the passivation of duplex stain-less-steel corrosion in concentrated hydrochloric acid solutions. Journal of Applied Electrochemistry, vol. 39. pp. 1385-1392. [ Links ]














