Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.5 Johannesburg May. 2015
PYROMETALLURGICAL PAPERS
Value-in-use model for chlorination of titania feedstocks
S. MaharajhI; J. MullerI; J.H. ZietsmanII
IExxaro Resources, Pretoria, South Africa
IIUniversity of Pretoria, Pretoria, South Africa
SYNOPSIS
In the chlorination process for TiO2 pigment production, blends of titania feedstocks such as ilmenite, synthetic rutile (sr), natural rutile, upgraded slag, and chloride-grade slag are reacted with coke and chlorine at a temperature of around 1000°C to form TiCl4 (the main product) and other waste metal chlorides. The TiCl4 is the main feed material for the TiO2 pigment-making process. Feeding different titania materials to the chlorinator affects the amount of coke and chlorine required for the process, the amount of waste generated, waste disposal costs, the amount of TiCl4 produced, and bed build-up rates. These factors influence the value of the feedstock. Generally, a higher TiO2 feedstock is more valued since less waste is generated and less reagents are consumed. To quantify the impact of different feedstocks on the chlorinator, a techno-economic model was developed to describe the chlorination process and estimate process variables at steady state. This paper describes the development of the model and studies in which the model has been used to quantify effects of using different feedstocks.
Keywords: titania feedstock, chlorination, process modelling TiCl4, value-in-use
Introduction
Approximately 90% of all TiO2 extracted from titanium-bearing minerals is used to produce white pigment (TZMI, 2012). A significant portion of this pigment is produced through the chloride process. This involves chlorination of TiO2 feedstocks such as natural and synthetic rutile, ilmenite, and high-titania slag in a fluidized-bed reactor to produce TiCl4, which is subsequently purified and oxidized to produce TiO2. The chloride process has stringent feedstock quality specifications to ensure that it can be operated in a stable and economical manner.
As a major TiO2 feedstock producer, Exxaro Heavy Minerals (now part of Tronox) needed to gain a thorough understanding of how their products would behave in their customers' chlorination reactors. This understanding would firstly assist in ensuring that an acceptable product is produced. Secondly, it would make it possible to gauge the value of the products in the hands of customers. To improve this understanding, it was decided to develop a techno-economic model of the chloride process that could be used to study the influence of different feedstock characteristics on the performance of the pigment production process.
Process analysis
This section presents details of the process analysis done with the purpose of collecting information that could be used as the basis of the modelling work.
Process description
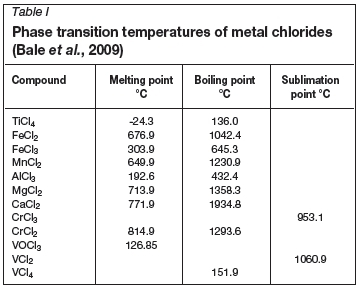
The purpose of the chloride TiO2 pigment production process is to extract the maximum amount of titanium from the TiO2-containing feed material in the form of titanium dioxide, while rejecting as much of the impurities (e.g. Ca, Mg, Si, Al, etc.) as possible to the waste streams, based on differences in the phase transition temperatures of different metal chlorides (Table I). An overview of the process is shown in Figure 1. The process consists of the following five stages (Lee 1991).
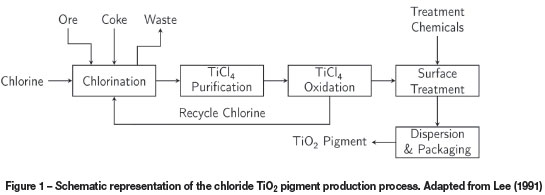
> Chlorination. This first stage is the focus of this paper. Chlorination converts feed materials to a solid waste stream and a crude liquid stream containing most of the titanium as TiCl4
> Purification. The crude TiCl4 produced in chlorination contains a wide range of impurities, which include solid iron and manganese chlorides, and unreacted ore, coke, and silicates, as well as soluble vanadium. Vanadium is converted to an insoluble chloride, and all the solid impurities are removed by vaporizing the TiCl4 and condensing it again
> Oxidation. Liquid TiCl4 from purification is vaporized and reacted with pre-heated oxygen to produce TiO2 and chlorine (Equation [1]). Chemicals are added for crystallization control, and the mixture of solids and gases is cooled before separation. The gas is recirculated to chlorination
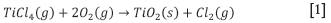
> Surface Treatment. The TiO2 particles are treated to improve their properties for specific pigment applications. This stage involves surface coating, washing, and de-watering to arrive at a final pigment product
> Dispersion and packaging. The final stage ensures that the product is in a suitable form and packaging for the intended application.
Details of the chlorination stage of the process are shown in Figure 2. The equipment consists of a continuous fluid bed chlorinator, a solids removal cyclone, a condensation unit, and a gas scrubber.
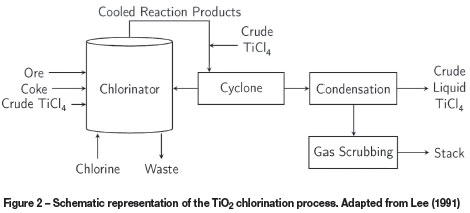
The chlorinator receives ore, coke, and chlorine as its main inputs. Some crude liquid TiCl4 is recycled to the chlorinator as a coolant for temperature control. Some of the solids blown over in the chlorinator are recovered by the cyclone and recirculated.
The combination of the cooled off-gas duct from the chlorinator and the solids removal cyclone is referred to as the 'cross-over and cyclone1. Recycled crude liquid TiCl4 is used as a coolant in the cross-over section. Cooling results in some of the chlorides precipitating as solids. The cyclone separates the coarsest solids from the vapour and fine solids. The coarse particles, which are a result of blow-over, are recycled back to the chlorinator.
The condensation unit splits the inlet gas stream into crude liquid TiCl4 and a gas stream. The gas is cleaned in the scrubber, and the crude TiCl4 is partially recycled and the remainder fed to the purification section.
The main chemical reactions occurring in the chlorinator are shown in Equations [2] to [5] (Lee, 1991).
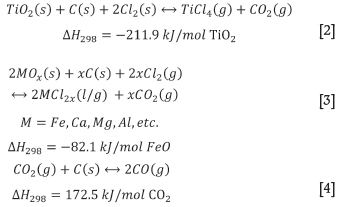
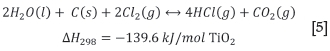
The chlorination reactor is operated at temperatures of around 1000°C. During chlorination most of the metal oxides are converted to chlorides and evaporated into the gas phase. High boiling-point chlorides such as CaCl2 and MgCl2 tend to remain in the bed as liquids, causing operational problems. SiO2 and ZrO2 tend not to chlorinate, and accumulate in the bed as solids. These problematic solids and liquids are bled from the bed to avoid build-up. Carbon binds to the oxygen in the metal oxides and leaves the reactor as a mixture of CO and CO2. Moisture tends to react with chlorine and carbon to form hydrogen chloride gas. The gaseous chlorides and other gas species leave the reactor and are cooled, causing most of the impurity metal chlorides to revert to the solid state, but leaving Ticl4 in the vapour phase (Lee, 1991).
The reactions occurring in the chlorinator are mostly exothermic. For example, when the reagents in Equation [2] are combined at 25°C adiabatically, the system would reach a temperature in excess of 1300°C. For this reason crude liquid TiCl4 is charged into the chlorinator as a coolant for temperature control. This input stream was not included explicitly in the model, but due to the assumption of isothermal equilibrium and the fact that separation efficiency is specified and not modelled, this has a negligible effect on the model results.
Material descriptions
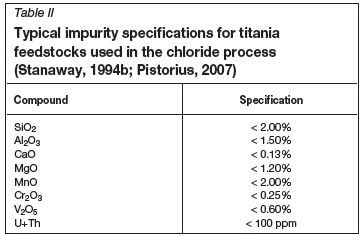
To operate the chlorinator efficiently, the TiO2 feed materials have to comply with stringent quality specifications (Stanaway, 1994b).
> The bulk density and particle size distribution must support fluidization of the bed, while minimizing blow-over of unreacted particles into the gas stream. The TiO2 feedstock particle sizes must typically be smaller than 850 μπι and larger than 100 μm. The bulk density of the material must be more than 2 kg/l
> The alkali oxide content (MgO and CaO) must be very low since the alkali chlorides are liquid at chlorinator operating temperatures, and can cause clogging of the bed
> Although most of the feed materials contain some iron, the iron content must be limited to minimize iron chloride waste generation and reagent consumption, and maximize plant capacity
> Chromium and vanadium can cause toxicity of the iron chloride waste. The levels of these elements in the feed must be limited
> Feed silica content must be limited to prevent build-up in the chlorinator
> Tin and arsenic tend to remain in the TiCl4 stream after purification, contaminating the final product. Feed materials must therefore be low in these elements
> Uranium and thorium are radioactive and concentrate in waste and product streams, causing health and safety risks. Very stringent specifications on these elements are enforced.
A typical chemical composition specification for high-TiO2 slag is shown in Table II.
Coke is ground to a particle size suitable for fluidization before use. This material also has to be of high purity to limit the introduction of impurity oxides into the process.
Industrial-grade chlorine is used in the main chloride feed stream. The recycled chlorine is less pure than the fresh chlorine.
Key phenomena
Given the purpose of the model, the most important process phenomena that had to be included were the following:
> Chemical reactions. The chemical reactions converting feed to product are the essence of the chlorination reactor. These are affected by both feedstock properties and operating conditions
> Blow-over. The entrainment of particles in the gas product stream from the chlorinator has an influence on the value of a particular feed material in the chlori-nation reactor
> Cyclone separation. The recovery of coarse particles in the cyclone and recycling to the chlorinator is of similar importance to blow-over.
Model development
The purpose of the model was to conduct techno-economic calculations to evaluate the influence of different feed materials and operating conditions on the behaviour and performance of the chlorination process. For this reason it was decided to employ a steady-state model based on the process mass and energy balance.
The equipment incorporated into the model included the chlorinator, the off-gas cooling duct and cyclone (cross-over and cyclone), and the condensation unit that separates the products into gas, liquid, and waste streams.
Model overview
The model flow sheet is presented in Figure 3. All solid material inputs to the chlorinator are split into two streams. A portion of the material is blown over based on particle size and density. The remaining solids are combined with the gaseous inputs and taken to isothermal thermochemical equilibrium to model the chemical reactions. The products are split into solid waste bled from the chlorinator and a gaseous product stream destined for the cross-over and cyclone.
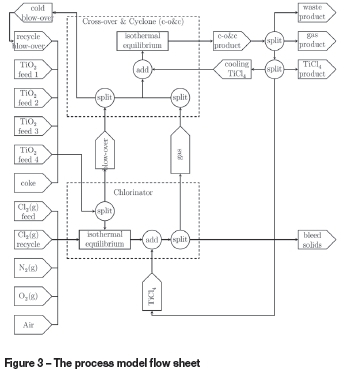
The blow-over solids and product gas from the chlorinator are charged into the cross-over and cyclone section. A portion of the blow-over solids is recovered and recycled to the chlorinator. The remaining solids and gas are combined with cooling TiCl4 and equilibrated to generate the final product stream from this section. The product from the cross-over and cyclone is split between waste solids, a gas product, and a crude liquid TiCl4 product.
Model variables and parameters
The material input variables supplied to the model by the user are listed in Table III, and the remaining input variables in Table IV. The model parameters are presented in Table V.
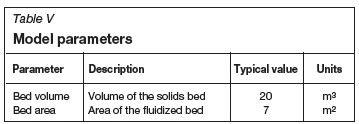
The material stream values calculated by the model are shown in Table VI. In addition to these variables, those listed in Table VII are also calculated.
Assumptions
The following assumptions were made in the model.
> The content of the fluid bed reactor is homogeneous in terms of temperature, particle size distribution, and chemical composition. This assumption is implicit in the lumped parameter modelling approach applied. The assumption is not true, but it does not have a detrimental influence on the modelling results, given the purpose of the model
> Chlorinator reactions run to equilibrium. This assumption and the next one were required to make it possible to solve the model. Very little detail was available on the actual chemical reaction behaviour of the process, since access to a plant was not possible. The model results based on this assumption were deemed to be acceptable for the purpose of the work
> Material reaches equilibrium in the cross-over
> Coke is assumed to be pure graphite. This assumption was made to simplify the model, and to focus it on the influence of the TiO2 feedstock rather than the coke. This means that sulphur is ignored by the model. Sulphur leaves the system through the waste gas system as sulphur species such as H2S, COS, and SOCl2. It has a limited influence on the chlorination phenomena of interest here
> Liquid phases are immiscible. For simplicity, all liquid compounds were handled separately as pure substances, and not as a mixture. This reduced the driving force for liquid formation because the activities of all the liquid compounds are unity. This assumption would have introduced inaccuracies into the equilibrium calculations
> Particles are compositionally homogeneous
> Chlorine slip is usually low and therefore was assumed to be zero in the model.
Chlorine slip occurs when chlorine passes through the chlorinator bed unreacted. This phenomenon reduces chlorine efficiency and indicates a chlorinator bed issue (e.g. high SiO2 content, low carbon content).
Material definitions
The materials in the model were described with thermo-chemical data from the FACT pure substance database in FactSage 5.5. The compounds included in the model are listed in Table VIII.
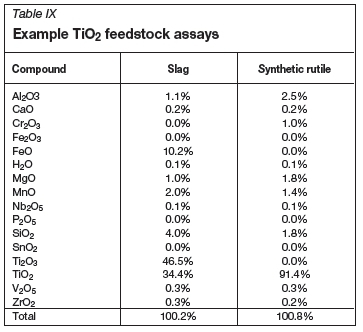
Examples of TiO2 feedstock assays are shown in Table IX. Coke, chlorine, oxygen, and nitrogen were all treated as pure streams. Air was entered as a 79% N2, 21% O2 mixture on volume basis.
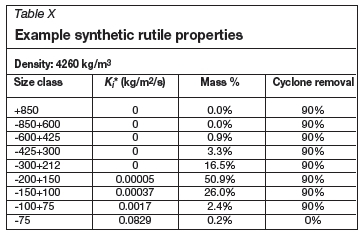
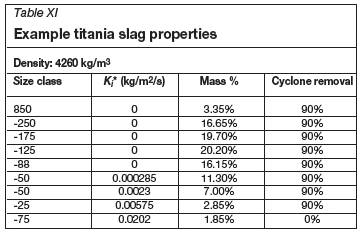
Some examples of particulate material properties for synthetic rutile and slag are shown in Tables X and XI.
Physical phenomena
Chemical reactions were modelled very simply through equilibrium calculations using the ChemApp thermochemistry library (Petersen and Hack, 2007), and data from FactSage (Bale et al., 2009).
Blow-over of solids in the chlorinator and subsequent recovery of coarse solids in the cyclone were the only physical phenomena that were modelled in detail, and are described here.
In the chlorinator, solids are entrained in the fluidizing gas and elutriated out of the chlorinator unit. This mass is significant and therefore had to be included in the model. Due to the complexities and uncertainties surrounding elutriation, a simplified methodology was used based on the following model input parameters for each feedstock material:
> Density - the solid density of the feedstock material
> Particle size distribution - the particles sizes and mass fraction of each size class for each feedstock
> Particle size elutriation constants (Ki) - the elutriation constants for each particle size class for each feedstock
> Particle size cyclone separation efficiency - the fraction of material of each particle size class recovered in the cyclone
> Composition - the composition of the feedstock material, which is assumed to be constant throughout each particle and to remain the same until it is blown out of the reactor.
In test work, the particulate solids elutriation constants are determined in units of kg/(s.m2). These constants are a function of particle shape, density, and size. This implies that for a reactor of a given area, a certain flux of solids of a specific particle size is expected in the outlet. Data on the fluidization of TiO2 feedstocks has been previously investigated (Moodley et al., 2012).
At steady-state operation the rate of particles blown over is limited to the incoming rate of particles of that size. For this system, the following are the sources of particles of a specific size:
> Feed material. Material feed is classified, and the particles of a certain average particle size enter at a specified total feed rate multiplied by the fraction of material with that average particle size
> Recycled blow-overfeed. Using the elutriation constant for a particular particle size class, the rate at which particles are blown over is calculated, with only some of these being removed in the cross-over and cyclone section. The blow-over rate is therefore multiplied with the separation efficiency in the cyclone for a particle size class to calculate the rate at which particles of a size class are recycled
> Larger size particles reacting. Chemical reactions in the chlorinator cause particles to shrink and hence fall into smaller size classes. This feed rate of particles into a lower size class is determined from the average particle sizes of the current size class and the larger size class, and the total rate of particles falling into the larger size class minus the rate blow-over rate.
Therefore, the blow-over rate of size class i (Equation [6]) is calculated as the minimum of the input rate of particles and the value calculated from the elutriation constant. This approach ensures that the blow-over rate is constrained by a mass balance over the chlorinator. The variable used in the blow-over and cyclone recovery calculations are defined in Table XII.
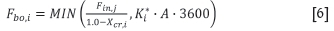
The first term in Equation [6], which is the maximum possible blow-over rate according to the mass balance, is derived as follows:
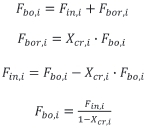
The rate at which particles enter size class i (Fin,i) is calculated as follows:
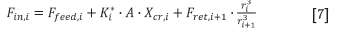
The rate at which particles leave the larger size class i+1 (Fou,i +1) due to chemical reaction is calculated as follows:

The total blow-over rate of a feedstock is calculated as the sum of blow-over for all the particle size classes.

The composition of the blow-over is derived from the composition of the feed materials. The above methodology is based on the following assumptions and simplifications:
> The composition of particles is that of the specified feed material, and is constant throughout each particle
> particles are spheres and are evenly distributed throughout each size class
> Particles do not break, and the only cause of particles becoming smaller is chemical reaction.
Implementation
The model was implemented mostly in the Microsoft .NET environment with the C# programming language. Microsoft Excel® was used as the front end because it is widely available and used in industry. All thermochemical calculations were done using the ChemApp thermochemical library (Petersen and Hack, 2007) and data exported from FactSage (Bale et al., 2009).
Validation
The model was validated qualitatively through interviews with persons that have first-hand knowledge and experience of the chlorination process. Quantitative validation was planned, but was ultimately cancelled for corporate reasons.
Modelling Studies
Value-in-use concepts
Value-in-use (VIU) models combine technical and financial information to provide a decision-making tool for product assessment and customer interaction. The concept aims to extract maximum sustainable value through knowledge and understanding of the value chain for the customer and producer. One of the main uses of VIU models is to evaluate product changes against a base case and determine the financial impact of the change. VIU models can also be used to compare and assess the value of different products in a customer's process. Examples where these two concepts have been practically used are provided in the following sections.
Example 1: production cost versus customer benefit
The first example evaluated the cost of producing a higher TiO2 grade slag versus the savings incurred by chloride producers.
Ilmenite (FeTiO3) is the most abundant titanium-bearing mineral and contains between 45 and 60% TiO2 (Moodley, 2011). Ilmenite cannot be used directly in most pigment production processes and has to be treated in order to upgrade the TiO2 content. In the smelting process, which can take place in either an AC or a DC furnace, ilmenite is reduced using anthracite to produce pig iron and titania-rich slag.
Titania slag competes with natural rutile (NR), synthetic rutile (SR), and upgraded slag (UGS) as feedstock to the chloride pigment process. NR, SR, and UGS contain more TiO2 units than the titania slag, which has a typical titania content of 85-87% TiO2. Production of a higher TiO2 slag using a conventional smelting process, although beneficial to the chloride pigment producers, incurs increased reductant and energy requirements, increased refractory wear, possible tapping issues, and foaming in the smelting furnace. There is an increasing demand for slag producers to produce higher TiO2 content slag, since waste generation and chlorine costs are reduced.
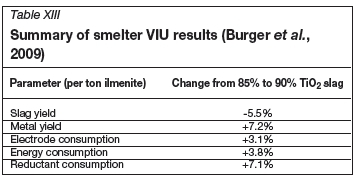
Burger et al. (2009) utilized the chlorinator model together with a smelter VIU model to evaluate the viability of producing a 90% TiO2 slag instead of the more conventional 85-87% TiO2 slag. The chlorinator model has been described in detail in this paper. The smelter VIU model was developed to model the production of TiO2 slag in a DC arc furnace. It is an Excel-based model that combines thermodynamic data and plant data to calculate the energy requirements, reductant requirements, and slag and metal yield for the smelter. This is combined with financial information to quantify the costs that the slag producer will incur to produce different quality slags.
Utilizing a higher TiO2 feedstock in the chlorinator results in less waste generation, lower treatment and disposal costs, and lower chlorine consumption, hence it results in a saving for chloride pigment producers. However, producing a higher TiO2 slag results in increased reductant and energy requirements, higher metal yield, and a lower slag yield. The chlorinator VIU model and a smelter VIU model were used to quantify the savings realized by the chloride producers and the extra costs incurred by the slag producer.
Smelter model assumptions
The following assumptions were made in the smelter model (Burger et al., 2009):
> All feed materials enter the process at 25°C
> Ilmenite feed composition from Tronox's Hillendale mine was used
> Reductant from Zululand Anthracite Collieries was used
> Recovery factors, dust loss rates, dust analyses ,and carbon contribution are derived from operating experience
> The carbon content of tapped iron is 2%
> The carbon efficiency factor is 94.5%.
Smelter results
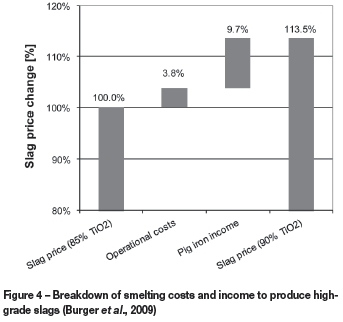
The results from the smelter VIU model are summarized in Table XIII. The base case was for the production of an 85% TiO2 slag. The results are expressed as the percentage change in various parameters when a 90% TiO2 slag is produced. Figure 4 is a waterfall graph that shows the increase in cost, expressed as a percentage of the slag price for a 85% TiO2 slag, due to the increase in operational costs (i.e. electrode consumption, energy consumption, and reductant usage). The decrease in income due to the changes in slag and metal yield is shown as 'pig iron income' in Figure 4. The VIU calculations indicate that slag producers will incur 13.5% cost increase in order to produce a 90% TiO2 slag using a conventional smelting process.
Chlorinator model assumptions
The following assumptions were made in the chlorinator model:
> All feed materials enter the reactor at 25°C
> Blow-over is determined by the elutriation constant, which was determined experimentally for different size fractions and different feedstocks
> Waste treatment includes neutralization of the waste with lime
> Chlorinator operating temperature is 1000°C
> The exit gas stream from the chlorinator is cooled to 200°C by liquid TiCl4 sprays (below the sublimation point of ferric chloride)
> The molar ratio of CO/CO2 in the gas product from the chlorination reactor is 1
> Price assumptions for the model are based on figures obtained from a TZMI (2007).
Chlorinator model results
In the base case, only an 85% TiO2 slag is fed to the chlorinator. The percentage change in reagent consumption and waste generation is reported in Table XIV for the alternative case, i.e. when only a 90% TiO2 slag is used in the chlorinator. A negative value indicates that consumption is lower with the 90% TiO2 feedstock.
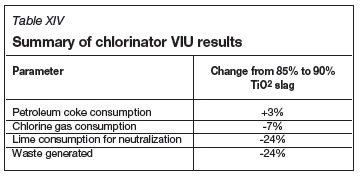
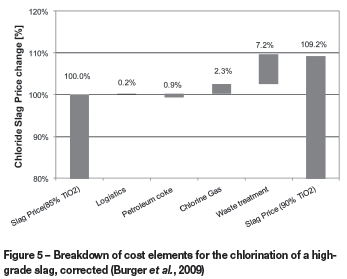
Figure 5 is a waterfall graph that shows the monetary contributions of coke, chlorine, logistics, and waste cost to the increase/decrease in value of the 90% TiO2 feedstock. The contribution is expressed as a percentage of the 85% TiO2 slag price (i.e. the base case). Chloride producers will utilize more coke to chlorinate a higher TiO2 feedstock, therefore petroleum coke will have the opposite effect of the chlorine and waste costs and reduce the value of the higher TiO2 feedstock as indicated in Figure 5. The impact of increased petroleum coke consumption is, however, low compared to the waste and chlorine savings. The overall VIU calculations indicate that the pigment producer's costs will be 9.2% lower if a 90% TiO2 slag is utilized instead of an 85% TiO2 slag.
The study indicates that the cost of producing a high-grade slag outweighs the savings realized at the pigment plant. However, although production of a higher TiO2 slag is not viable based on the assumptions and prices used in the model, this could alter as prices change, and the use of VIU models will enable the changes to be quickly assessed.
Example 2: relative feedstock values
In the second example, the relative value of an 86% TiO2 feedstock is compared with a 95% TiO2 feedstock. Utilizing a higher TiO2 feedstock in the chlorinator results in less waste generation, lower treatment costs, lower disposal costs, and lower chlorine consumption, and hence results in a saving for chloride producers. The value of TiO2 feedstock containing more TiO2 units should therefore be higher. The chlorinator model was used to determine the relative value of using a titania slag (86% TiO2) compared to a natural rutile product containing 95% TiO2 in the chlorination process, considering the major cost elements (i.e. the costs of petroleum coke, chlorine, waste disposal and waste treatment).
Model assumptions
> Same as in Example 1
> Price assumptions are based on figures obtained from a TZMI (2012) and escalated to present-day prices.
Model results
The major results from the chlorinator VIU model are provided in Table XV. In the base case, only an 86% TiO2 slag is fed to the chlorinator. The percentage change in reagent consumption and waste generation is reported in Table XV for the alternative case, i.e. when only natural rutile is used.
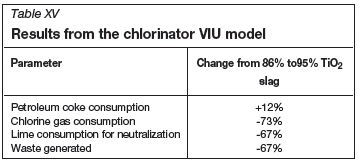
With natural rutile, less waste is generated, and less lime and make-up chlorine is required, but coke consumption increases.
The waterfall graph (Figure 6) shows the monetary contribution of coke, chlorine, and waste cost to the increase/decrease in value of the natural rutile. The contribution is expressed as a percentage of the 86% TiO2 slag price (i.e. the base case).
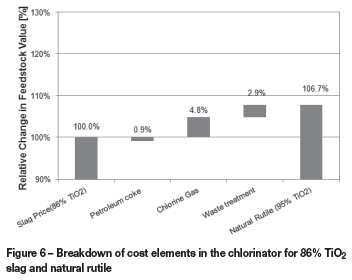
VIU calculations show that for the given set of assumptions and prices, the value of natural rutile in the chlorinator is 6.7% higher than that of the slag. This is largely due to the chlorine costs, which account for 4.80% of the 6.7% change. In the Burger et al. (2009) study, the contribution from waste treatment costs (Figure 5) was the highest, but due to the decrease in waste treatment costs (TZMI, 2012), the chlorine costs are now the major cost driver.
Knowledge of the relative value of the feedstocks is important for suppliers to position themselves in the market and understand the value of their feedstock.
Conclusions
Modelling of the chlorination process facilitates the quick assessment of different feedstocks and provides valuable insight into a process that feedstock producers normally do not have access to.
Although VIU models are a powerful-decision making tool, care must be taken to ensure that the assumptions are valid and are regularly updated.
References
Bale C. W., Bélisle, E., Chartrand, P., Decterov, S.A., Eriksson, G., Hack, K., Jung, I. H., Kang, Y.B., Melanqon, J., Pelton, A.D., Robelin, C., and Petersen, S. 2009. FactSage thermochemical software and databases -recent developments. Calphad, vol. 33. pp. 295-311. www.factsage.com [ Links ]
Burger, H., Bessinger, D., and Moodley, S. 2009. Technical considerations and viability of higher titania slag feedstock for the chloride process. 7th International Heavy Minerals Conference, 'What Next'. Champagne Sports Resort, Drakensberg, South Africa, 20-23 September 2009. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 187-194. [ Links ]
Hamor, L. 1986. Titanium dioxide manufacture. Australia: A World Source of Ilmenite, Rutile, Monazite and Zircon Conference, Perth, Australia. Australasian Institute of Mining and Metallurgy. pp. 143-146. [ Links ]
Kotzé, H., Bessinger, D., and Beukes, J. 2006. Ilmenite smelting at Ticor SA. Southern African Pyrometallurgy Conference, Cradle of Humankind, South Africa, 5-9 March 2006. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 203-214. [ Links ]
Lee, R.F. 1991. Chloride route titanium dioxide pigments. Process and properties. Fifth AusIMM Extractive Metallurgy Conference. Perth, Australia, October 1991. Australasian Institute of Mining and Metallurgy. pp. 35-38. [ Links ]
Moodley, S. 2011. A study of the chlorination behaviour of various titania feedstocks. MSc thesis, University of the Witwatersrand, Johannesburg. [ Links ]
Moodley, S., Kale, A., Bessinger, D., Kucukkaragoz, C., and Eric, R.H. 2012. Fluidization behaviour of various titania feedstocks. Journal of the Southern African Institute of Mining and Metallurgy, vol. 112, no. 6. pp. 467-471. [ Links ]
Petersen S. and Hack, K. 2007. The thermochemistry library ChemApp and its applications. International Journal of Materials Research, vol. 98, no. 10. pp. 935-945. [ Links ]
Pistorius, P.C. 2007. Ilmenite smelting: the basics. 6th International Heavy Minerals Conference 'Back to Basics', Hluhluwe, South Africa, 9-14 September 2007. Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 35-43. [ Links ]
Stanaway, K.J. 1994a. A titanium pigment feedstock overview. SME Annual Meeting, Albuquerque, New Mexico, 14-17 February 1994. pp. 1-6. [ Links ]
Stanaway, K.J. 1994b. Overview of titanium dioxide feedstocks. Mining Engineering, vol. 46. pp. 1367-1370. [ Links ]
TZMI. 2007. Global TiO2 Pigment Producers - Comparative Cost and Profitability Study. TZ Minerals International Pty Ltd, Victoria Park, WA, Australia. [ Links ]
TZMI. 2012. Global TiO2 Pigment Producers - Comparative Cost and Profitability Study. TZ Minerals International Pty Ltd, Victoria Park, WA, Australia. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2015. ISSN2225-6253. This paper was first presented at the, Pyrometallurgical Modelling Principles and Practices, 4-5 August 2014, Emperors Palace Hotel Casino Convention Resort, Johannesburg.














