Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.5 Johannesburg May. 2015
PYROMETALLURGICAL PAPERS
The recovery of platinum group metals from low-grade concentrates to an iron alloy using silicon carbide as reductant
W. MalanI; G. AkdoganI; S. BradshawI; G.A. BezuidenhoutII
IUniversity of Stellenbosch Process Engineering Department, South Africa
IILonmin Western Platinum Limited, Process Division, Marikana, South Africa
SYNOPSIS
The purpose of the study was to investigate the feasibility of SiC reduction of low-grade concentrates from Lonmin's Rowland and Easterns operations with respect to metal fall and PGM recovery. These concentrates are rich in SiO2 and MgO with low concentrations of chalcopyrite and Cr2O3. Pd is the most abundant of the PGMs. SiC reduction of samples was conducted at 1600°C with 2.5-3.5 kg SiC per 100 kg concentrate. PGM recoveries for Easterns concentrate were better than for Rowland. More than 85% of the Ir and Pd and almost 60% of the Pt were recovered with 3.5 kg SiC per 100 kg concentrate. SEM of slag samples showed little entrainment of metallic prills compared to Rowland samples. This was attributed to the relatively higher melt viscosities of the Rowland concentrate. In order to decrease slag viscosity and to enhance PGM recovery, the FeO content of the Easterns concentrate was increased with the addition of 10 kg converter slag per 100 kg concentrate. Ir and Pd recoveries were increased to about 95%, while Pt recovery was around 70%. On the basis of these results an optimum feed ratio between Easterns and Rowland concentrates and converter slag is proposed. Carbothermic reduction of the optimum charge was also compared to SiC reduction. Carbothermic reduction yielded a marginally higher metal fall; however, the calculated gas emissions and energy requirements were higher than for SiC reduction.
Keywords: SiC reduction, PGM recovery, LG concentrates, FactSage modelling
Introduction
South Africa is the world's leading supplier of platinum group metals (PGMs). Most of the PGMs are contained in the Merensky and UG2 reefs of the Bushveld Complex, where they are associated with nickel- and copper-bearing minerals. In Merensky ore, the major base-metal sulphide mineral is pyrrhotite, with pentlandite, chalcopyrite, pyrite, and minor amount of other sulphides also present. In UG2 (Upper Group 2) ore the major base-metal sulphide is pentlandite. Pyrrhotite is found in moderate amounts, and millerite and pyrite in minor amounts. Because of lower mining costs, platinum mining is becoming more UG2-based, and the resulting concentrates contain high levels of chromite unless blended with Merensky ore. It is therefore critical that new and improved extraction methods be developed and exploited. The methods used in the recovery of the PGMs from these ores consist of physical concentration techniques, pyrometallurgical processing, and hydrometal-lurgical extraction of the base metals followed by the PGMs (Jones and Kotze, 2004; Nell, 2004).
During pyrometallurgical processing, the nickel-copper concentrates from the mill-float concentration step are smelted to bring about physical and chemical changes that enable recovery of base metals, PGMs, and other valuable metals in crude form. In general, the idea is to melt the concentrate in a furnace to produce a matte, which contains all the sulphides, below a carefully maintained slag layer. This matte, which contains large amounts of iron and sulphur, is oxidized in a Peirce-Smith converter to lower the iron and sulphur levels, while at the same time increasing the PGM grade. Conventional PGM matte smelting essentially requires the presence of a certain quantity of base metal sulphides in order to collect the PGMs in a molten sulphidic phase in the smelting furnace. However, the quantity of chrome oxide in the feed materials (particularly UG2) need to be strictly controlled to avoid the build-up of high-melting chromite spinels (Jones and Kotze, 2004; Nell., 2004).
Currently, the matte-based collection process is most widely used for PGM recovery, but because PGM-containing concentrates are becoming more enriched with UG2 concentrates, it is expected to be integrated with or replaced by an alternative processes. Mintek has developed an alternative process for smelting PGM-containing oxide feed materials that contain low sulphur levels, and often high levels of chromium oxide, known as the Conroast process. The process involves the smelting of dead-roasted sulphide concentrates in order to generate a small amount of an iron alloy in which PGMs and base metals are collected. A slag is also produced, which contains mostly unwanted materials and very low levels of residual PGMs. The desired degree of reduction is controlled by adjusting the carbon addition. Highly reducing conditions promote high recoveries of the valuable metals to the alloy, but high iron collection dilutes the PGM grade of the alloy, which affects the PGM recoveries in the converting and refining processes (Jones and Kotze, 2004; Nell J., 2004).
In this study, low-grade (LG) concentrates from Lonmin's Rowland and Easterns operations were smelted with SiC as reductant. The effect of process parameters such as reductant to concentrate ratio, temperature, and different reductants on metal fall, alloy composition, slag composition, and gas composition were investigated. FactSage modelling was also used to simulate the reduction process. Detailed chemical and mineralogical characterization of the feed, alloy, and slag was conducted by X-ray diffraction (XRD), X-ray fluorescence (XRF), scanning electron microscopy (SEM), and inductively coupled plasma (ICP) analysis.
The primary objectives of this study include understanding the reducing conditions to produce the minimum amount of alloy while maintaining a high as possible recovery of PGMs; quantifying the deportment of the various elements to the alloy and slag phases, and establishing the factors affecting the recovery of PGMs; and comparing the gas emissions from different reductants in terms of environmental impact. The benefits of the new process would include no constrain on the minimum quantity of base metals required in the feed material, as the PGMs are collected in an iron-based alloy; ability to integrate the relatively large quantities of the alloy product into an existing smelter complex, possibly through a converter; allowing for the possibility of hydrometallurgical refining of the alloy; chromium tolerance; efficient PGM collection; fewer sulphur emissions compared to current matte-smelting processes; and possibly an economic incentive for treating other waste materials in a similar manner.
Materials and methods
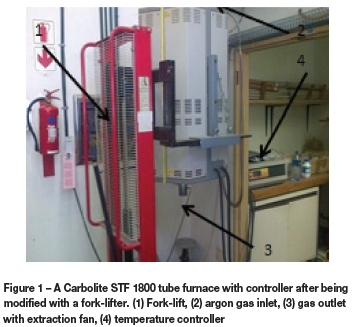
A Carbolite STF 1800 tube furnace with a programmable Carbolite controller was used for the experiments. The alumina tube was heated by five surrounding lanthanum elements. A set point of 1600°C was set on the controller and the measured temperature recorded by a thermocouple located in the furnace. The initial plan was to attach a crucible to a wire and then suspend it from the top of the furnace at the tube outlet and lower it to the marked hot spot. However, in order to facilitate the operation, the furnace was removed from its original supporting brackets and fitted onto a fork-lifter. After the adjustment, the furnace could easily be moved up and down in vertical direction. The movement was carefully controlled by a hydraulic lever or foot pedal (Figure 1). After the furnace had reached the set temperature, the MgO crucible containing the sample mixture was placed into the tube furnace. Before the sample was placed into the hot zone, high-purity argon was allowed to purge into the Al work tube at a flow rate of 5 l/min for 20 minutes. The sample was then raised to the hot zone, and the Ar flow rate was reduced to about 600 ml/min at the start of the experiment. After a predetermined time of reduction, the furnace was raised and the sample was allowed to cool rapidly under an Ar flow rate of 5 l/min for about 10 minutes.
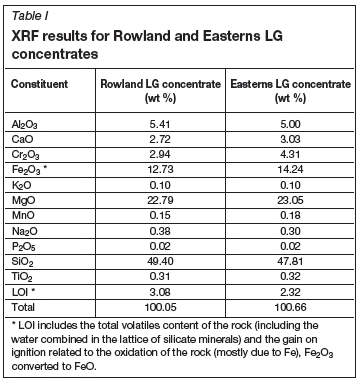
The screen analysis showed that P80values for Easterns concentrate, Rowlands concentrate, and SiC reductant were 320 μm, 180 μm, and 380 μm respectively. The chemical compositions of the LG concentrates are given in Tables I and II.
Results and discussion
FactSage simulations
Initial FactSage simulations were conducted prior to laboratory-scale experiments in order to establish a range of optimum conditions. Thermodynamic equilibrium results are given in 100 kg concentrate per mass of reductant. The stoichiometric amount of SiC required per 100 kg of LG concentrate is 0.27 kg for Rowland and 0.38 kg for Easterns. These were determined as the minimum amounts of SiC required to produce a molten metal phase, primarily being Fe (> 98%), given the starting oxide concentration and ease of reduction. Experimental results are then compared with FactSage results and discussed. Without any reductant in the system, MgCr2O4(s) spinel accumulates, therefore a reductant mass of 3 kg SiC per 100 kg LG concentrate was chosen as a base case. During the prediction of optimum temperature range, the requirement was that Fe concentrations should be high, while Cr and Si should remain low. The respective liquidus temperatures during SiC reduction are 1420°C for Rowland and 1410°C for Easterns concentrate. The results indicated that Fe and Cr concentrations in the alloy should not be significantly affected when the temperature increased from 1400°C to 1650°C, while Si concentrations would increase. The solubility of C in the alloy decreases with increasing temperature. The metal fall is not significantly affected, however, and about 1600°C should yield a good metal fall and avoid formation of solid phases like MgSiO3(s) proto-enstatite. Therefore 1600°C was selected as the temperature at which to investigate the SiC reduction of the LG concentrates.
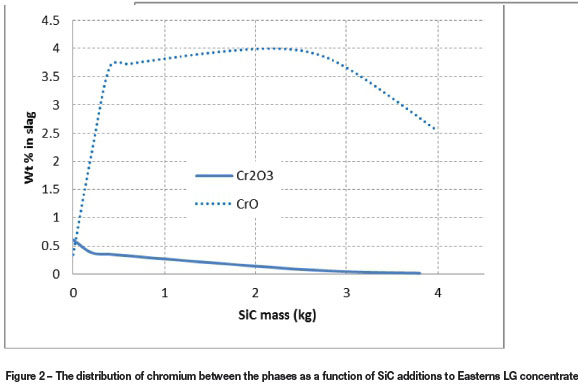
The effect of reductant to concentrate ratio was investigated from 0.5 kg to 4 kg SiC per 100 kg LG concentrate. It was found that an increase in reductant mass will cause more oxides, particularly FeO, to reduce, thereby increasing metal fall. For both concentrates a molten metal phase starts to form when 0.5 kg of reductant is present per 100 kg concentrate, emphasizing the ease of reduction of both concentrates, and almost all Cr2O3 is reduced to CrO, thereby increasing the solubility of Cr in the slag phase. The metal fall continues to rise and it therefore becomes easier to recover PGMs. Better metal fall for reductive smelting of Easterns LG concentrate is expected at higher reductant to concentrate ratios, due to the marginally higher FeO concentration. The molten metal phase consists primarily of Fe and other easily reduced base metals; however, some Cr and Si deport to the metal phase once the SiC to concentrate ratio is increased. Si and Cr start to dissolve in the molten metal at significant concentrations when the reductant mass exceeds 3 kg SiC per 100 kg LG concentrate. C concentrations in the alloy also become more significant at a higher SiC to concentrate ratio. Cr solubility in the slag phase is greatly affected by the oxidation state of Cr (Nell, 2004; Jones, 2009). Chrome in its trivalent state (spinel) has a very low solubility in the slag phase - divalent chrome, in contrast, is highly miscible in the slag phase (Nell, 2004). The FactSage simulation in Figure 2 shows how Cr is reduced from Cr(III) to Cr(II) as function of SiC additions to Easterns LG concentrate charge.
Experimental results
An alloy from each experiment was separated from the slag phase and weighed. The percentage metal fall for each experiment was determined as the amount of alloy formed per amount of initial concentrate. The results showed that metal fall was increased by a longer residence time (from 60 minutes to 180 minutes) and greater reductant to concentrate ratio. Some thermogravimetric analysis (TGA) experiments were also conducted to investigate the reaction mechanisms and kinetics. The alumina pedestal with the attached crucible containing the LG concentrate charge and SiC reductant were placed on a scale, which was connected to a computer with a VGA cable to acquire the change in mass of the concentrate charge. It was found that the mass of the crucible, refractory support material, and alumina pedestal remained constant throughout the experiment. Therefore the only change in mass was that of the concentrate and reductant charge in a gas-tight furnace. The mass changes on the scale were noted every 0.5 seconds on the computer, and typical graphs of mass loss vs time were constructed. The results showed that no significant mass changes occurred after about 10 minutes of reduction time, indicating that equilibrium was reached due to the reduction of iron oxides, given their relatively high concentration in the original concentrates. It is known that the iron oxide, FexOx, will be reduced before the base metal oxide, CrxOx (Chakraborty et al. 2010; Haque and Ray, 1995). The results from thermodynamic modelling also agreed with this finding that FeO reduction needs to be near completion for CR2O3 reduction to commence. If it is assumed that Fe and Cr make up the bulk of the alloy, given the relatively low concentration of Ni- and Cu-bearing minerals, the reactions will take place in the following order:
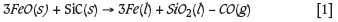
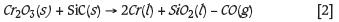
Andrews (2008) also concluded that a longer residence time will improve the kinetics of matte droplet settling. Furthermore, increasing the amount of SiC causes more FeO to be reduced to Fe, causing more metal prills to form and increasing the probability of coalescence. This, once again, agrees well with previous work (Shahrokhi and Shaw, 2000; Saffman and Turner, 1956; Ammann et al., 1979). The chemical compositions of the alloys obtained from SiC reduction of Rowland and Easterns LG concentrate are shown in Tables III and IV respectively.
Fe forms the bulk of the overall composition of all of the alloys from SiC reduction of Rowland LG concentrate. This correspond well with work done by Perry et al. (1988) and Qayyam et al. (1976), who also concluded that reduction of FeO will take place before the reduction of CrO. Cr concentrations increase sharply when the reductant to concentrate ratio is increased. When the reductant to concentrate ratio is 3.5 kg SiC per 100 kg Rowland LG concentrate, Cr constitutes almost 10% in the alloy. Cr concentrations are more than 5% in alloys from tests with different reduction times. These high concentrations of Cr could cause difficulties during downstream processing, particularly during converting and in the base metals and precious metals refineries. Ni and S concentrations seem to increase with residence time. These two elements most likely originate from the mineral pentlandite. Pt and Pd concentrations remain very low in all of the alloys.
Easterns LG concentrate has a higher FeO content than Rowland LG concentrate, which explains the higher Fe content of alloy samples produced from reduction of Easterns LG concentrate. The same observation was made from the FactSage modelling. The low Cr and Si concentrations in the alloy are very favourable for downstream processing because of the difficulties in removing these elements during converting and refining. The Pt and Pd concentrations are higher in alloys from SiC reduction of Easterns LG concentrate. The better metal fall is the most likely factor to have contributed to the higher PGM concentrations in these alloys. PGM recoveries (Ir, Pd, and Pt) to the alloy phase for the respective concentrates are shown in Table V and Table VI.
From Table V it is seen that the recoveries of Ir, Pt, and Pd are very low. The highest Pt and Pd recoveries weres achieved at a reductant to concentrate ratio of 3 kg SiC per 100 kg Rowland LG concentrate and residence time of 180 minutes. One factor that may contribute significantly to the low PGM recoveries is slag viscosity. The recoveries of Ir, Pd, and Pt from SiC reduction of Easterns LG concentrate are significantly better (Table VI). Ir, Pd, and Pt recoveries increase significantly when the quantity of reductant is increased. Overall, Ir has the highest recovery, followed by Pd and Pt. The very low Cr and Si concentrations in these alloys will allow for a further increase in reductant quantity and therefore it should be possible to recover more than 90% of PGMs by SiC reduction of Easterns LG concentrate.
The slag compositions from the SiC reduction of Rowland and Easterns LG concentrates are shown in Tables VII and VIII respectively.
The results from Tables VII and VIII show a significant decrease in FeO concentration compared with the initial concentrates. The lowest FeO content corresponded to the experiments with the highest quantity of reductant. The composition of the slag has a major effect on the viscosity, and FeO, NiO, CuO, and CrO will all aid in lowering the viscosity. Once the SiC comes in contact with these oxides, it will reduce and settle to join the alloy phase, thereby removing them from the slag phase and increasing the viscosity. It can be seen that the reduction process lowered the FeO concentration compared with the initial concentrate (refer to Table I).
The viscosity module in FactSage was used to determine the viscosities at 1600°C. At a temperature of 1600°C it can be safely assumed that the slag is molten. The calculated viscosities of the slags from SiC reduction of Rowland and Easterns LG concentrate at the beginning of the melts are 3.74 and 3.06 poise, respectively. The significantly higher slag viscosity for Rowland LG concentrate would make it more difficult for small metal prills to settle and coalesce.
Table IX shows viscosities of the slags at the end of the melt. The viscosities were calculated in the FactSage viscosity module from the slag compositions in Table VII and Table VIII.
Table IX shows that the calculated viscosities of the slags from all the tests are higher than the calculated viscosities at the beginning of the melts. This is because the bulk of the Fe has partitioned into the alloy. Some Cr has also deported to the alloy phase. The viscosities of slags from SiC reduction of Rowland LG concentrate are significantly higher than slags from SiC reduction of Easterns LG concentrate. This could explain why the PGM recoveries from Rowland LG concentrate are significantly lower.
Viscosities of PGM smelting slags are reported in the literature (Eric, 2004; Eric and Hejja, 1995) as being in the range 1.5-4 poise, which could hinder the settling of particles 15 μm or smaller in size. The FeO/SiO2 ratio of the slag seems to have a major influence on the viscosity. The Rowland LG concentrate under investigation has a lower FeO/SiO2 ratio than Easterns LG concentrate. When FeO is reduced, slag viscosity will increase and valuable metal prills will not settle. The high initial slag viscosity will contribute to insufficient coalescence of metal prills and phase separation. The corresponding viscosities from these experiments conducted with Rowland LG concentrate fall mostly above the range of 1.5-4 poise. It therefore seems that high slag viscosities are the primary reason for the low PGM recoveries. Slag viscosities from SiC reduction of Easterns LG concentrate do, however, fall in the recommended range. The lower viscosities will aid in the coalescence of metal prills, enhance phase separation, and induce good PGM recovery.
Figures 3 and 4 are SEM images of the slag phases from SiC reduction of Rowland and Easterns LG concentrate respectively, from experiments that had the same reductant to concentrate ratio and reduction time.
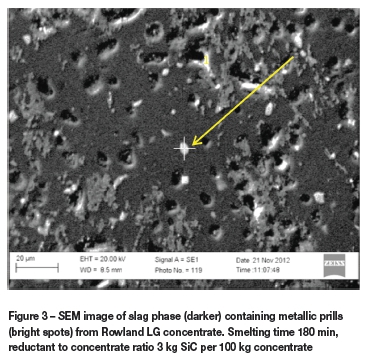

Figure 3 shows a SEM image of the slag phase from SiC reduction of Rowland LG concentrate. Metal prills are entrained in the slag. These metal prills are very small and are most likely trapped in the slag phase and will never settle or coalesce with other metal prills of similar size. Studies in the literature indicate that many small metal prills (< 2 μm) are expected never to settle under the force of gravity alone. This phenomenon agrees well with the work done by Fagurland and Jalkanen (1999) and Poggie et al. (1969), who also reported that very small droplets may either settle very slowly or may be trapped as 'rafts' of droplets floating on the top surface of the slag or as droplets suspended below small gas bubbles. However, the high viscosity of the slag is most likely restricting the coalescence and settling of metal prills. An EDX spot analysis on the metal prill indicated by the arrow detected Ir and Pd.
Figure 4 shows a SEM image of the slag phase from SiC reduction of Easterns LG concentrate. The slag phase is smoother, with no metal prills entrained. This is an indication that metal prills coalesced and settled well within the slag phase. From these findings it is very clear that the lower slag viscosity contributes significantly to PGM recovery from SiC reduction of Easterns LG concentrates. This corresponds to similar findings in the literature (Shahrokhi and Shaw, 2000; Saffman and Turner, 1956; Ammann et al., 1979).
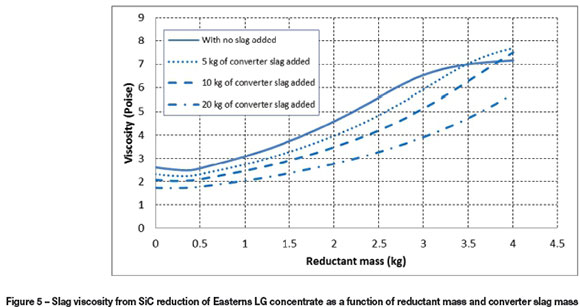
From the results obtained experimentally, it was concluded that Easterns LG concentrate should form the bulk of the dry LG concentrate feed to a DC arc furnace, since it has a higher FeO/SiO2 ratio, which is significant in reducing slag viscosity and increasing overall PGM recovery. The slag viscosities need to be kept in the region of approximately 3.2 poise (average viscosity determined from experiments with SiC reduction of Easterns LG concentrate) for acceptable PGM recoveries. Rowland and Easterns concentrates could be combined, with the main fraction being Easterns, or another source added that would lower slag viscosity. The slag from a Peirce-Smith converter has a high FeO/SiO2 ratio and could be used to reduce the slag viscosity. FactSage was used to model such a scenario and an experiment was also conducted to back up the results. FactSage modelling was conducted at 3.5 kg SiC per100 kg Easterns LG concentrate at 1600°C with varying amounts of converter slag additions.
Figure 5 shows that addition of converter slag does decrease the slag viscosity. The converter slag is rich in FeO and thereby the FeO/SiO2 ratio in the slag is increased, causing the slag viscosity to decrease. However, the converter slag contains about 30 wt% SiO2, which could act to increase the slag viscosity. Figure 5 shows that an increase in reductant will cause more FeO to be reduced, consequently a system with 5 kg and 10 kg converter slag additions will have a higher slag viscosity than a system with no converter slag if the reductant additions are increased past the point of intersection. Another critical point is that the conductivity and viscosity of the slag are inversely related. A decrease in viscosity will increase the conductivity of the slag; increasing iron oxide serves to depolymerize the slag and creates sites for electronic conduction (Nell, 2004; Eric, 2004). It is clear from the findings that around 10 kg of converter slag addition per 100 kg concentrate would decrease slag viscosity without increasing the total quantity of slag significantly. The alloy is also expected to have a good PGM grade. A test was therefore conducted to compare the results from experiments and FactSage modelling for similar conditions.
From Table X, it is apparent that FactSage predicts a slag with similar composition. The marginally lower FeO/SiO2 ratio in the slag from FactSage modelling will cause the viscosity to be somewhat higher than the slag from the experiment. Table X also shows an improved PGM recovery with converter slag addition, which is likely to be a result of the decrease in slag viscosity. The Pd and Ir recoveries are nearly 100%, compared to Pd and Ir recoveries of 94.5 and 86.6% obtained experimentally with no slag addition. Pt recovery has also improved, from 59.2% to almost 70%. Pt recovery is expected to improve if the metal fall increases. More reductant could be added to increase metal fall and thereby increase the Pt recovery from the initial concentrate. Overall, this is this is a significant finding. A portion of converter slag can therefore be recycled and re-melted together with any LG concentrate to increase metal fall and PGM recoveries.
FactSage modelling was used to predict an optimum ratio between Rowland and Easterns LG concentrates. The reductant to concentrate ratio was fixed at 3.5 kg SiC per 100 kg LG concentrate and the temperature at 1600°C. FactSage predicted that adding more than 30 kg converter slag per 100 kg LG concentrate decreases the alloy to slag ratio. Therefore a 0.7-0.8 fraction of Easterns LG concentrate in the feed and 20-30 kg converter slag per 100 kg LG concentrate should not significantly increase furnace slag quantities and the FeO/SiO2 ratio should be adequate to sustain a slag viscosity close to 3.5 poise. At these operating conditions more than 90% of the PGMs should be recovered from a LG concentrate feed in an alloy with a high PGM grade. Cr and Si concentrations in the alloy are less than 1% in total, and C concentrations are less than 1%.
FactSage modelling was also undertaken to compare SiC reduction with carbon reduction. The optimum feed conditions, namely 75% Easterns LG concentrate, 25% Rowland LG concentrate, 25 kg converter slag per 100 kg LG concentrate, and 0-4 kg reductant per 100 kg LG concentrate at 1600°C were used in the comparison. With these conditions, it is assumed that slag viscosity will be low enough to ensure good phase separation and PGM recovery. The results indicated that C reduction of a LG concentrate charge results in a marginally higher metal fall. The alloy compositions were very similar and only small differences in Fe, Cr, and Si concentrations were noted. Gas emissions and energy requirements are higher for C reduction, arguably due to C reacting endothermically with FeO to produce Fe(l) and CO(g) in contrast to SiC reacting exothermically with FeO to produce Fe(l), SiO2(l) and CO(g).
However, it must be borne in mind that SiC is not a naturally occurring compound and is manufactured by carbothermic reduction of silica at high temperatures, which entails its own high level of emissions which should be added to the emissions from SiC smelting. A preliminary FactSage analysis was conducted for the reaction to produce SiC:
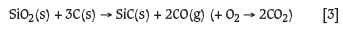
The resulting estimates of energy requirements and CO emissions per ton of SiC product are given in Table XI. It is seen from Table XI that not all of the SiO2 and C has reacted to form SiC. Thermodynamic modelling predicts that a small fraction of the SiO2 will be converted to SiO(g), leaving some unreacted C(s) in the system. Overall more than 1.3 t of CO (g) is emitted to produce 1 t of SiC. Moreover, the energy required to produce the given SiC quantities will contribute to overall energy requirements for recovery of PGMs from an iron alloy. This needs to be taken into consideration from an environmental and economic point of view when considering a reductive smelting route. It was, however, determined that only approximately 35 kg SiC per ton of LG concentrate is required to recover most of the PGMs. It must also be taken into consideration that these PGMs need to be extracted from a more enriched LG concentrate feed, and reductive smelting is the only feasible option at the present time.
Conclusions
The feasibility of using SiC as reductant for Rowland and Easterns LG concentrates was investigated with respect to metal fall, PGM grade in the alloy, slag composition, and PGM recovery, using small-scale experiments in combination with FactSage modelling. The findings can be summarized as follows.
> SiC reduction of Easterns LG concentrate resulted in significantly better metal fall and PGM recoveries compared with Rowland concentrates. At a reductant to concentrate ratio of 3.5 kg SiC per 100 kg Easterns LG concentrate, more than 85% of Ir and Pd were recovered, and more than 60% of Pt. The higher FeO/SiO2 ratio of Easterns LG concentrate and consequent lower slag viscosity is most likely to have contributed to the improvement of recoveries. SEM images from a slag from SiC reduction of Easterns LG concentrate showed no entrained metal prills, indicating that most metal prills had coalesced and settled. Cr and Si concentrations were below 2% in total in all alloys
> In order to improve the PGM recoveries, the FeO content of the initial charge had to be increased. Peirce-Smith converter slag from Lonmin was used as an addition to increase FeO/SiO2 ratio. The experiments resulted in more than 95% recoveries for Ir and Pd, together with about 70% for Pt. FactSage modelling predicted that Easterns LG concentrate should make up 70-80% of the LG concentrate charge and 20-30 kg converter slag be added per 100 kg LG concentrate to attain relatively lower viscosities for phase separation. It is expected that PGM recoveries of more than 90% should be obtained from the LG concentrate, in conjunction with a good PGM grade in the alloy
> Using the optimum LG concentrate charge, the effectiveness of SiC as a reductant was also compared to that of C through thermodynamic modelling. C reduction of a LG concentrate charge led to a marginally higher metal fall at the same reductant to concentrate ratio compared with SiC reduction. The alloy composition was very similar and only small differences in Fe, Cr, and Si concentrations were noted. Gas emissions and energy requirements were higher for C reduction of a LG concentrate charge.
> From the evidence, it is clear that SiC reduction seems a reasonably attractive alternative to carbon-based reductants. Therefore, integrating this process into the present matte-based collection flow sheet could be considered as a future alternative for smelting LG concentrates. However, it must be borne in mind that SiC is not a natural compound and it should be manufactured by again carbothermic reduction of silica at high temperatures with its own level of emissions. This obviously will require further investigation.
Acknowledgements
The authors wish to thank Lonmin Plc for the financing of this work.
References
Andrews, L. 2008. Base metal losses to furnace slag during processing of platinum-bearing concentrates. MSc thesis, Faculty of Engineering, University of Pretoria. [ Links ]
Chakraborty, D., Ranganatha, S., and Sinha, S. 2010. Carbothermic reduction of chromite ore under different flow rates of inert gas. Metallurgical and Materials transactions B, vol. 41B. pp. 10-18. [ Links ]
Eric, R.H. and Hejja, A.A. 1995. Dimensioning, scale up and operating considerations for six electrode electric furnaces. Part 2: Design and scale-up considerations for furnaces treating PGM-containing copper-nickel concentrates. EPD Congress, Warren, G.W. (ed.). The Minerals, Metals & Materials Society, Warrendale, PA. pp. 239-257. [ Links ]
Eric, R.H. 2004. Slag properties and design issues pertinent to matte smelting electric furnaces. Journal of the South African Institute of Mining and Metallurgy, vol. 104, no. 9. pp. 499-510. [ Links ]
Fagerlund, K.O. and Jalkanen, H. 1999. Some aspects on matte settling in copper smelting. Fourth International Conference Copper 99 - Cobre 99, Phoenix, Arizona, 10-13 October 1999. Volume VI. Society for Mining, Metallurgy, and Exploration, Englewood, CO. pp. 539-551. [ Links ]
Haque, R. and Ray, H.S. 1995. Role of ore/carbon contact and direct reduction in the reduction of iron oxide by carbon. Metallurgical and Materials Transactions B, vol 26B, no.2. pp. 400-401 [ Links ]
Jones, R.T. and Kotze, I.J. 2004. DC arc smelting of difficult PGM-containing feed materials. First International Platinum Conference, 'Platinum Adding Value', Sun City, South Africa, 3-7 October 2004. South African insitute of Mining and Metallurgy, Johannesburg. pp. 33-36. [ Links ]
Jones, R.T. 2009. Towards commercialisation of Mintek's Conroast process for platinum smelting. Pyrometallurgy of Nickel and Cobalt. 48th Annual conference of Metallurgists of CM, 2009. pp 159-168. [ Links ]
Nell, J. 2004. Melting of platinum group metal concentrates in South Africa. Journal of the South African insitute of Mining and Metallurgy, vol. 104, no. 7. pp. 423-428. [ Links ]
Poggie, D., Minto, R., and Davenport, W.G. 1969. Mechanisms of metal entrapment in slags. Journal of Metals, November 1969. pp. 40-45. [ Links ]
Shahrokhi, H. and Shaw, J.M. 2000. Fine drop recovery in batch gas-agitated liquid-liquid systems. Chemical Engineering Science, vol. 55. pp. 4719-4735. [ Links ]
Saffman, P.G. and Turner, J.S. 1956. On the collision of drops in turbulent clouds. Journal of Fluid Mechanics, vol. 1. pp. 16-30. [ Links ]
Ammann, P.R., Kim J.J., and Loose, T.A. 1979. The Kennecott Process for nickel slag cleaning. Journal of Metals, vol. 1, no. 2. pp. 20-25. [ Links ]
Perry, K.P.D., Finn, C.W.P., and King, R.P. 1988. An ionic diffusion mechanism of chromite reduction. Metallurgical Transactions B, vol. 19B. pp. 677-684. [ Links ]
Qayyam, M.A. and Reeve, D.A.A. 1976. Reduction of chromites to sponge ferrochromium in methane-hydrogen mixtures. Canadian Metallurgical Quarterly, vol. 15, no. 3. pp. 193-200. [ Links ]
© The Southern African Institute of Mining and Metallurgy, 2015. ISSN2225-6253. This paper was first presented at the, Pyrometallurgical Modelling Principles and Practices, 4-5 August 2014, Emperors Palace Hotel Casino Convention Resort, Johannesburg.














