Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.4 Johannesburg Apr. 2015
STUDENT EDITION
Air drying of fine coal in a fluidized bed
M. Le Roux; Q.P. Campbell; M.J. van Rensburg; E.S. Peters; C. Stiglingh
School of Chemical and Minerals Engineering, North-West University, Potchefstroom, South Africa
SYNOPSIS
The demand for energy has continued to rise worldwide in line with population growth. The majority of South Africa's electricity is supplied by coal-fired power stations. The amount of fine coal (-2 mm) generated at coal processing plants has increased, due mainly to mechanized mining methods. Fine coal retains more water, which lowers its heating value.
Drying the coal is costly and it is difficult to achieve the required moisture content. Consequently, coal fines are often discarded. An estimated 8% of the total energy value of mined coal is lost1.
Fluidized bed technology is often used to dry coal thermally, but this method is expensive and has an adverse environmental impact. The objective of this study was to investigate the removal of moisture from fine coal (<2 mm) in a fluidized bed operated with dry fluidizing air at moderate temperatures as the drying agent. The effects of different air temperatures and relative humidity levels were investigated in a controlled environment. The study further investigated the influence of coal particle size on moisture removal.
The drying rate was found to increase with increasing temperature. The relative humidity of the drying air had a more pronounced effect on the drying rate, even at temperatures as low as 25°C.. It became more challenging to remove moisture as the particle size decreased. The gain in calorific value was greater than the energy required to dry the coal samples, showing that a fluidized bed using moderately warm dry air is an energy-efficient drying technology. The energy efficiency of the fluidized bed compared favourably with other thermal drying methods.
Keywords: coal fines, drying, fluidized bed, energy efficiency.
Introduction
The increased use of mechanized coal mining methods has resulted in greater amounts of coal fines being generated. Many operations report an estimated 6% of their ROM production to be in the -2 mm size range. Fine and ultra-fine size ranges constitute about 11% of the nominal product and retain the bulk of the moisture (SANEDI, 2011). Given the problems associated with moisture in fine coal, it is important to investigate and improve available moisture removal techniques. Coal constitutes the primary source of energy in South Africa and is a major contributor to the economy, and therefore improving coal quality will in effect maximize the quantity of usable coal (De Korte and Mangena, 2004). With the use of effective dewatering methods, fine coal can be benefi-ciated and added to the coarse particle circuits without compromising the quality of the product and hence substantially increasing the overall plant yield (Condie and Veal, 1998). Condie and Veal (1998) suggest that by rule of thumb, for every ton of moisture removed the clean coal product stream is supplemented with about 4 t of fine coal. This is a powerful incentive for developing advanced dewatering techniques for fine coal particles. Additionally, excessive moisture adds to the mass-based transport costs of coal. For this reason, developing advanced efficient fine coal drying techniques is beneficial from an economical point of view (Campbell, 2006).
Rowan (2010) states that coal preparation plants generally discard coal fines with size fractions below 150 µm into waste ponds. This poses a danger of spontaneous combustion, acid mine drainage, and dust release as the surface of the coal is exposed to ambient air and weathering conditions for long periods (De Korte and Mangena, 2004). Coal fines are more susceptible to water absorption than coarser coal and can contain up to 25 wt% total moisture after filtration (Le Roux, 2003). Thermal drying methods are more efficient than mechanical dewatering techniques (De Korte and Mangena, 2004), but the price of coal limits the use of these methods (SANEDI, 2011). Studies conducted at North-West University showed that drying of fine coal (-2 mm +1 mm) in a fluidized bed is possible at low temperatures between 25°C and 40°C.
Work by Le Roux et al. (2012) on vacuum filtration showed that intentionally damaging a filter cake improved the airflow infiltration, leading to a lower pressure differential across the cake but increasing the dewatering efficiency. This work confirmed that high airflow conditions resulted in a lower final cake moisture content of 3-5 wt%. Further studies found that an increased airflow rate resulted in a more effective moisture transfer from the coal fines to the drying air (Le Roux et al., 2013).
The moisture content of fine coal particles is made up of surface, capillary, and chemically bound moisture (Rong, 1993) as depicted schematically in Figure 1. Free moisture is found on the exterior surface of coal particles (Condie and Veal, 1998), and can be removed by mechanical methods such as filters and centrifugal units. Capillary-bound moisture is absorbed and held tightly within micro-capillaries and micropores of individual coal particles (Rong, 1993). Removal of this moisture calls for thermal drying techniques for complete drainage (Condie and Veal, 1998). Chemically bound moisture is not included when measuring the total moisture content of the coal (Campbell, 2006), and can be removed only by pyrolysis.
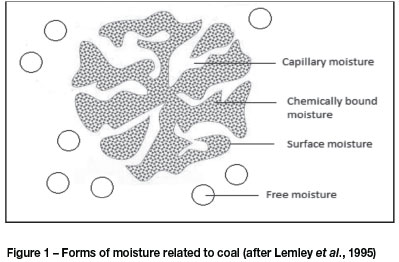
The equilibrium moisture content of coal is characterized as the moisture content at which the coal particles no longer gain or lose moisture, and it varies according to the temperature and relative humidity conditions of the atmosphere surrounding the particles. Mechanical methods are insufficient for the removal of this equilibrium moisture, which can be reduced only by means of evaporation (Le Roux et al., 2013). The relative humidity and temperature act as driving forces that change the phase equilibrium between vapour and liquid, with lower humidities and higher temperatures leading to moisture being absorbed from the particle by the drying medium (Koretsky, 2004).
Experimental method
The aim of this project was to determine the effect of temperature, relative humidity, and particle size distribution on the efficiency of drying fine coal particles in a fluidized bed. The energy consumption during the drying process was calculated and compared to published data on thermal drying processes.
Sample preparation
South African bituminous coal from the Waterberg coalfield was used for these experiments. The proximate analysis of this type of coal is given in Table I. The coal was crushed and sieved into three particle size ranges: fines (between 2 mm and 1.18 mm) and ultra-fines (between 1.18 mm and 0.5 mm). The samples were drenched in water for a day and the excess free moisture was removed by pressure filtration. The moisture content of each filtered sample was determined (SANS5925:2007) before the coal was fed to the fluidized bed for dewatering.
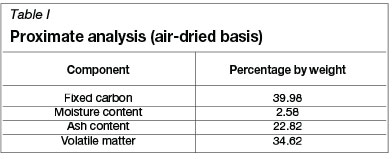
Apparatus
A fluidized bed column (10 cm inner diameter x 40 cm length) was constructed from polycarbonate (Figure 2). The column was connected to a blower, which was used to draw conditioned air at a set temperature and relative humidity from a climate chamber (CTS climate test chamber Type: C-40/100). A packed bed of glass marbles in the bottom section of the fluidized bed acted as airflow distributor. Mesh covers (0.5 mm aperture) were placed at the top and bottom sections of the fluidized bed to retain the bulk coal sample within the cylinder. The outlet air from the column was returned to the climate chamber, and was recirculated to the column after the temperature and relative humidity values attained the pre-set levels. For each test, 100 g of fine coal sample with a total moisture content of approximately 25-35 wt% (typical of a pressure filter product) was fed to the fluidized bed cylinder. The weight of the column was continually monitored during fluidization to determine the loss of moisture. A number of selected experiments were repeated in the fluidized bed to determine the repeatability of the results.
Results and discussion
Influence of temperature
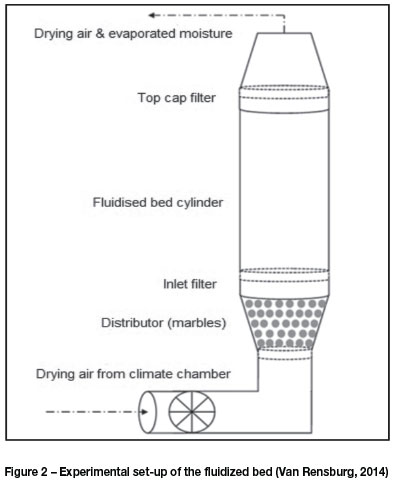
To study the effect of air temperature, wet samples of 100 g containing about 30 wt% total moisture were placed in the fluidized bed cylinder, and drying air was introduced at a superficial velocity of 1.5 to 1.7 m/s, which was slightly above the predetermined minimum fluidization velocity. Two sets of experiments were conducted at air temperatures of 25°C and 55°C respectively and a relative humidity (RH) of 30%. Figure 3 shows the moisture loss from the ultra-fine sample (-1.18 mm +0.71 mm) under these conditions. The drying rate was quicker at 55°C than at 25°C. The drying time was reduced from 28 minutes to 21 minutes. This confirms the observation made by Rowan (2010) that elevated temperatures lead to higher dewatering rates. Higher temperatures disrupt the phase equilibrium and increase the amount of water transported from the coal sample into the surrounding air (Condie and Veal, 1998).
Duplicate experimental runs proved the repeatability of the results, the maximum and minimum standard deviation being 3.40 wt% and 0.12 wt% respectively.
Influence of relative humidity
For the next set of experiments, the fluidizing air was introduced at relative humidities of 30%, 50%, and 70% at a constant temperature of 55°C. The drying curves are shown in Figure 4. A comparison of Figure 4 with Figure 3 shows that relative humidity has a greater effect on the drying rate than temperature. Lower relative humidities counteract the capillary forces retaining the moisture in the coal particle, leaving a dried product in about 14 minutes at 30% RH, compared to over 30 minutes for 70% RH at the same temperature. Higher relative humidities weaken the moisture transfer mechanism, and therefore the moisture is displaced from the capillary channels at a lower rate (Condie and Veal, 1998). Van Rensburg (2014) stated that the mechanism for the transfer of water molecules from the coal to the air is enhanced when the drying air contains low moisture levels. This leads to a high transfer rate of the water molecules from an area of high moisture content to an area of low moisture content.

Influence of particle size
Three wet filter cake samples, all with an initial moisture content of 33 wt%t and different size ranges (-1.18 mm +0.71 mm, -0.71 mm +0.50 mm, and a 50/50 mixture of -1.18 mm +0.71 mm and -0.71 mm +0.5 mm), were dried in the fluidized bed using feed air at 55°C and 50% RH. Figure 5 shows the drying curves for these experiments.
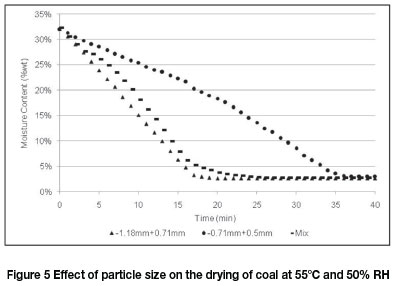
The coarse fraction (-1.18 mm +0.71 mm) and the 50% mixture showed similar drying responses, reaching a final moisture value in less than 20 minutes, while the finer fraction (-0.71 mm +0.5 mm) reached a similar final moisture value only after 37 minutes. This shows that it is increasingly difficult to remove moisture as the size of the coal particles decreases. Small particles have large surface areas and more micropores to absorb water, resulting in a higher degree of water retention (De Korte and Mangena, 2004).
Drying rates
Figure 6 shows the different drying rates for fluidizing feed air conditioned at 25°C across the set relative humidity ranges. A decrease in relative humidity clearly increases the drying rate for all particle size ranges. The drying rate increased from 0.010 wt%/min to 0.015 wt%/min for the -2 mm +1.18 mm particles at 25°C with 70% and 30% RH respectively. It is also apparent that the drying rate increased with an increase in
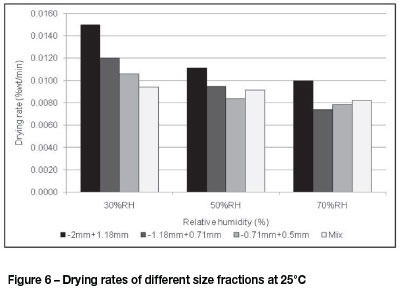
particle size at less than 50% RH. It is noteworthy that at 25°C, particle size is not the rate-limiting factor at relative humidity conditions exceeding 50% RH. The -0.71 mm +0.5 mm fraction had the lowest drying rate at 25°C and 70% RH, while the fastest drying rate was for the -2 mm +1.18 mm particle size range at 30% RH.
It is clear that relative humidity has a more significant effect on the drying rate of the coal particles than temperature, hence dry air at moderately low temperatures is effective in drying fine coal.
Energy consumption
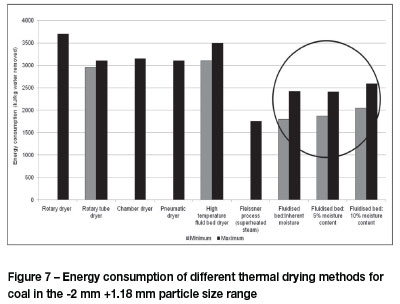
The energy required during the drying process was calculated by considering the change in enthalpy of the water, the work done by the blower, as well as the energy required for the conditioning of the air. A temperature of 25°C and relative humidity of 50% were chosen as a basis for the calculations, since these were the average ambient conditions in the laboratory. The calorific value of the coal was upgraded by 8 MJ/kg on average using air at these conditions as drying medium. Figure 7 shows the calculated maximum and minimum of energy requirements to dry to three different moisture levels using the fluidized bed with those of other existing thermal drying technologies. It can be seen that fluidization is more energy-efficient than other thermal processes, with the exception of the Fleissner process.
Conclusion
This work has shown that fine and ultra-fine coal particles can be dried at moderate temperatures and low relative humidities in a fluidized bed. The time required for fines is about half of that required to dry ultra-fines. The main driving force for removal of moisture from fine and ultra-fine coal is relative humidity. Energy calculations demonstrate that fluidization is more energy-efficient than other thermal drying processes. Using dry air at a moderate temperature in a fluidized bed to dry coal particles is thus a promising technique warranting further study and development, since it has a potential energy advantage as well the ability to increase the calorific value of the coal.
Acknowledgements
The authors would like to acknowledge the following institutions for their contribution towards this project:
➤ Coaltech
➤ NRF (National Research Foundation).
This work is based on research supported by the South African Research Chairs' Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Any opinion, finding, or conclusion, or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard.
References
Campbell, Q.P. 2006. Dewatering of fine coal with flowing air using low pressure drop systems. PhD dissertation, North-West University, Potchefstroom. 130 pp. [ Links ]
Condie, D. and Veal, C. 1998. Improved fine coal dewatering via modelling of cake desaturation. CSIRO, Australia. pp. 1-34. [ Links ]
De Korte, G.J. and Mangena, S.J. 2004. Thermal Drying of Fine and Ultra-fine Coal. Report no. 2004 - 0255. Division of Mining Technology, CSIR, Pretoria. pp. 5-24. [ Links ]
Karthikeyan, M., Zhonghua, W., and Mujumdar, A.S. 2009. Low-rank coal drying technologies - current status and new developments. Drying Technology, vol. 27, no. 3. pp. 403-415. [ Links ]
Koretsky, M.D. 2004. Engineering and Chemical Thermodynamics. 2nd edn. John Wiley & Sons Hoboken, NJ. [ Links ]
Le Roux, M. 2003. An investigation into an improved method of dewatering fine coal. Master's dissertation, North-West University, Potchefstroom. 96 pp. [ Links ]
Le Roux, M., Campbell, Q.P., and Smit, W. 2012. Large-scale design and testing of an improved fine coal dewatering system. Journal of the Southern African Institute of Mining and Metallurgy, vol. 112, no. 7. pp. 673-676. [ Links ]
Le Roux, M., Campbell, Q.P., and Van Rensburg, M.J. 2013. Fine coal dewatering using high airflow. International Journal of Coal Preparation and Utilization, vol. 34. pp. 220-227. [ Links ]
SANEDI (South African National Energy Development Institute). 2011. http://www.sanedi.org.za/coal-roadmap/ [Accessed 25 June 2014]. [ Links ]
Rong, R.X. 1993. Literature review on fine coal and tailings dewatering. Advances in Coal Preparation Technology, vol. 2. Project P239A. JKMRC, University of Queensland. Brisbane. Australia. 120 pp. [ Links ]
Rowan, S.L. 2010. Analysis and scaling of a two-stage fluidized bed for drying of fine coal particles using shannon entropy, thermodynamic exergy and statistical methods. PhD dissertation, University of West Virginia, Morgantown WV. 154 pp. [ Links ]
Van Rensburg, M.J. 2014. Drying of fine coal using warm air in a dense medium fluidised bed. Master's dissertation, North-West University, Potchefstroom. 98 pp. [ Links ]
Yu, A.B., Standish, N., and Lu, L. 1994. Coal agglomeration and its effect on bulk density. Powder Technology Journal, vol. 82, no. 1. pp. 177-189. [ Links ]
Paper written on project work carried out in partial fulfilment of Degree in Chemical Engineering (NWU) - Pursuing Masters in Coal Beneficiation
1 Rowan, S.L. 2010. Analysis and scaling of a two-stage fluidized bed for drying of fne coal particles using shannon entropy, thermodynamic exergy and statistical methods. PhD dissertation, University of West Virginia, Morgantown WV.














