Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.4 Johannesburg Apr. 2015
STUDENT EDITION
Evaluation of some optimum moisture and binder conditions for coal fines briquetting
P. Venter; N. Naude
University of Pretoria
SYNOPSIS
Coal mining is a thriving industry and 53% of the coal mined in South Africa is used for electricity generation. Mechanization has made coal mining more efficient, but fines generation has subsequently increased. Up to 6% of the run of mine material can report to the -200 µm fraction. Common problems associated with fines handling include dust formation, storage problems, and high moisture levels. A method to turn this material into a saleable product instead of stockpiling it can add value to a company.
Briquetting is a pressure agglomeration method where loose material is compacted into a dense mass (FEECO International, 2014). The briquettes must be able to withstand rigorous handling and transport operations without disintegrating. This study aims to investigate the optimum binder and moisture conditions required to produce a mechanically strong briquette using two different binders - a PVA powder (binder A) and a starch powder (binder B).
It was found that for binder A the optimum moisture level was 12% to 14%. At this moisture level the greatest compression strength gains were observed, and low amounts of fines produced in impact and abrasion tests. The minimum amount of binder added while still obtaining a strong briquette was 0.5% binder A. For binder B the optimum moisture level was also 12% and the minimum amount of Binder B to be added was found to be 1%. Briquettes that were dried outside reached their peak strength after about four days, whereas the briquettes that dried inside took about 20 days to reach their strength plateau. Hardly any degradation took place on the surface of the binder A film after exposure of 300 hours of artificial weathering. Thermogravimetric analysis confirmed that neither binder A nor binder B will add to the ash content of the coal fines, as both binders totally decompose above 530°C.
Binder B yielded stronger briquettes after 15 days and also generated less fines. It is therefore superior to binder A and would be recommended for further use.
Keywords: coal fines, briquetting, binder, moisture level.
Introduction
The need for coal briquetting
The coal mining industry in South Africa has been operating for more than 120 years. Annually, 224 Mt of coal is produced, of which 25% is exported. The remainder is used to feed South Africa's industry, with 53% used for generating electricity (Eskom, 2014). Electricity generated from coal-fired power stations accounts for 77% of South Africa's electricity supply.
Fines generation in coal mining has increased as a result of mechanized mining, and up to 6% of the run of mine product can be in the -200 µm fraction. Coal-fired power stations in South Africa do not accept a product of -200 µm size because of the high moisture content(England, 2013). In this paper, any -8 mm material will be considered as fines. Additional problems arising from coal fines generation include flow problems from containers, dust formation in plants and fire hazards during stockpiling.
An increased moisture content inevitably reduces the calorific value of the coal, as well as increasing handling problems. Instead of pumping these fines to slime dams or discarding them in old workings, a means of economical agglomeration can be beneficial to mines and power stations.
ESI Africa (2014) estimates the amount of thermal-grade fines stockpiled over the past 100 years at about 1 billion tons. Only in the last few years have methods to utilize these stockpiles in South Africa started to be explored. These methods include briquetting, pelletizing, and granulation.
Briquetting is a pressure agglomeration method where loose material is compacted into a dense mass (FEECO International, 2014). It is a more advanced and more expensive process than pelletization and granulation, but the end-product can withstand the rigorous handling methods that export coal undergoes, and in some cases, is water-resistant.
Table I shows that, assuming that Eskom will pay a similar price per ton of briquettes to that of coarse coal, it is not financially viable to produce briquettes using a generous amount of binder. Producing briquettes with a modest amount of binder look more promising. According to Sastry et al., binder cost may represent 60% of the total cost of briquette manufacture. However, since low binder additions can be detrimental to the mechanical strength of the briquettes, a compromise must be reached between binder content and mechanical strength.
A review of previous work done on briquetting
The earliest coal briquettes were made in hand-filled brick moulds using clay and cow dung as binders. These bricks had poor mechanical properties which made them unsuitable for transportation over long distances. Only by the 1850s were mechanical methods introduced to briquette brown coal and lignites without the use of binders, while hard coal briquettes required binders to stay intact.
Roller presses were first developed in Belgium by Louiseau to address the need for strong briquettes. Pillow-shaped holes in the roller faces compact material into dense briquettes that weighs no more than 50 g each. The basic principles of these machines have remained relatively unchanged over the years apart from small improvements to extend the life and reduce maintenance.
The briquetting process is conducted at room temperature and the use of binders depends on the coal grade being used. The complete briquetting process and binder options are discussed later in this paper.
Binders for coal briquetting
It is possible to use binderless pelletization for coal; however, most coal fines are not self-agglomerating. The literature suggests numerous binders for the pelletization of coal (Altun et al., 2001; Dehont, 2006):
➤ Coking and oil refining residues such as tar and coal pitch
➤ Residues from paper mills (lignosulphonate)
➤ Molasses with possible additions of lime
➤ Starch
➤ Synthetic resins
➤ Synthetic polymers.
These binders should have adequate binding strength, relatively low cost, and be resistant to weathering (Waters, 1969).
Briquetting process
The main operations during a briquetting process are as follows (Waters, 1969):
➤ Screening and drying of the coal (if too wet)
➤ Mixing of coal with binder
➤ Feeding to briquette machine and pressing
➤ Drying
➤ Storing and packaging.
According to Dehont (2006), the size distribution of the coal fines should be roughly as follows:
➤ 50% from 0 to 0.5 mm
➤ 25% from 0.55 to 1 mm
➤ 20% from 1 to 2 mm
➤ 5% from 2 to 3 mm.
The fines should have a low moisture content, good compatibility, small particle size, and a wide particle size distribution to facilitate good packing of the particles. Proper mixing is also critical to ensure that the binder is distributed evenly throughout the mixture.
Physical testing of briquettes
According to Richards (1990), the most important physical properties of briquettes are resistance to crushing, impact, abrasion, and water penetration. These properties are all heavily dependent on the development of strong and durable bonds between particles during the agglomeration stage. It is critical that briquettes withstand storage, handling, and transport during which they will be dropped, experience abrasion on conveyors, and be exposed to the elements. Four laboratory tests are recommended to monitor the physical strength properties of briquetted fuels either during process development or commercial production. These are a drop shatter test, a crushing resistance test, a tumbler abrasion test, and an immersion water resistance test (Richards, 1990).
Method
Coal fines from Exxaro's Mafube coal mine near Middelburg were used in the briquetting process. This study forms part of research by Exxaro into coal fines agglomeration, and the binders that were used were prescribed by Exxaro. Two binders were used - a PVA powder (binder A) and starch powder (binder B). Coal fines from Exxaro's Mafube coal mine near Middelburg were used in the briquetting process.
Ten days of testing were allocated for physical tests, ranging from day 0 to say 35. 'Day 0' refers to the day that the briquettes were manufactured, as these briquettes have not been allowed to dry for a full day.
The mechanical properties of the briquettes were investigated by means of following tests.
Compressive strength
Compressive strength is the maximum crushing load a briquette can withstand before cracking or breaking. A single briquette was placed on the platform of the tensile strength testing machine and, with the machine operating in the compressive mode, a constant load was applied until the briquette fractured.
The load at fracture can also be converted to a stress using the equation:

By expressing the load as a stress (force per unit area) it is possible to compare briquettes of various sizes and incorporating different binders. For the purpose of this study all briquettes were of similar size and shape, and therefore only the load force was used. A batch of 20 briquettes was tested at a time.
Impact resistance
A batch of 20 briquettes was dropped once from a height of 2 m, and the particle size analysis of the pellets and broken pieces conducted. According to Richards (1990), 'Impact resistance testing is considered to be the best general diagnostic of briquette strength'.
Abrasion resistance
A charge consisting of 20 briquettes was rotated in a tumbler machine for 100 revolutions at 50 revolutions per minute. The tumbler drum had dimensions of 278 mm in length by 20 mm in diameter, and a 38 mm wide lifter plate was also welded along the length of the drum. The charge was then collected and the particle size distribution (PSD) of the fines (-8 mm) that were generated was calculated.
Water resistance
Since briquettes may in some cases be stockpiled outside and exposed to the elements, it is important to test for water resistance. A single weighed briquette was submerged in a beaker of cold tap water and inspected for disintegration by applying finger pressure at intervals of about ten minutes. If the briquette remained intact after 30 minutes, the surface water was wiped off with a cloth and the briquette was weighed again. To obtain a quantitative comparison, a water resistance index (WRI) was calculated as follows:
WRI = 100-%water after 30min
Richards (1990) argues that a WRI > 95% should be obtainable after 30 minutes
Artificial weathering of binder A
A QUV Accelerated Weathering Tester was fitted with A340 UV lamps. Binder A was exposed to alternating cycles of UV light and elevated temperatures. The temperature was set to 63°C and the irradiance at 0.67 W/m2, and the samples were run on a dry cycle.
After exposure of only a few days the QUV tester can reproduce damage that will take months or years outdoors.
The rate of polymer oxidation was measured by conducting infrared spectroscopy (IR) on the exposed films. By following the growth of the carbonyl peak near 1720 cm-1, a carbonyl index was defined by the ratio of this absorption to that at 2900 cm-1 and used to quantify the progression of degradation.
Thermogravimetric analysis
The sample was heated to a maximum temperature of 900°C, and the residual mass plotted against temperature. The material that remains after the maximum temperature is reached should correspond to the total ash percentage of the coal sample.
Results and discussion
Compressive strength
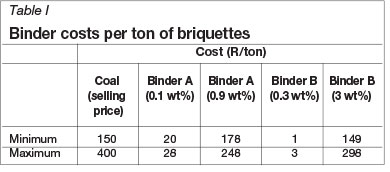
The SABS 1399:1999 standard was used to ensure the briquettes met the minimum compressive strength requirements. This standard specifies the requirements for charcoal made from wood in either lump or briquette form. The compression strength of the briquettes was first evaluated as a function of moisture content (the total moisture of the batch during the briquetting process). From Figure 1 it can be seen that the compressive strength increased from day 0 to day 15 for each moisture level. At 10% moisture level, briquettes with binder B, with an initial strength of 37 N, gained hardly any strength after 15 days. Binder A yielded an increase of only about 40 N after 15 days. This low moisture content does not allow for efficient dispersion of the binder throughout the coal fines. Briquettes with 12% moisture showed the greatest strength gains for both binder A and binder B. Day 0 compression strength results could not be obtained from briquettes with 18% moisture and using binder B, as they were too soft and disintegrated under the load.
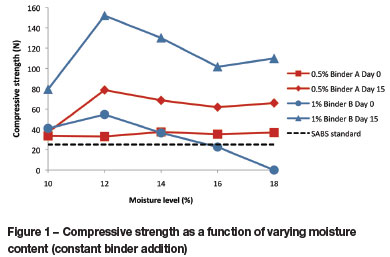
In Figure 2 the strong dependence of compressive strength on binder content is illustrated very clearly. The initial strength, as well as day 15 strength, increases with increase in binder content. Briquettes with binder B at levels of 0.3% and 0.5% show poor strength, and attain strength values well above the SABS standard of 25 N only with binder additions of 1% and more. The maximum strength of 358 N was obtained on day 15 for 3% binder B. However, it may be necessary to make a trade-off between the strength and binder cost. Lower additions of binder B result in acceptable briquette strengths, with gains of 61 N and 97 N for 1% and 2% binder B addition respectively.
Low levels of binder A also resulted in weak briquettes. Only at a level of 0.5% binder A and higher is sufficient compression strength achieved in the briquettes. The highest binder A addition of 0.9% also resulted in the greatest strength gain of 68 N. This is followed by 0.5% binder A with a gain of 46 N, which will be a more economical option.
The observed trend of increasing compressive strength with increased binder addition is expected because at higher binder levels more binder is available to be dispersed between the coal particles and ensure bonding between binder and coal.
Drop and tumble tests
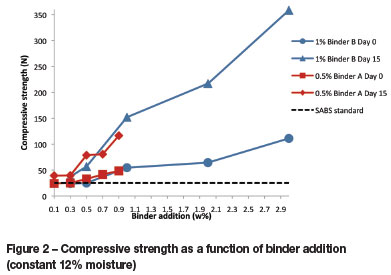
The results of the drop and tumble tests were combined on a single axis for easier comparison. In Figure 3 the binder addition is constant, with moisture as the variable. SABS standard 1399 specifies a maximum of 5% fines. Fines are considered to be material that passes through an 8 mm screen. The tumble test produced more fines than the drop test for all moisture contents. For day 0 testing, when the briquettes are at their weakest, the briquettes with binder A generated on average 24% fines, while for binder B this was significantly less at 8%.
For both binders, the greatest amount of fines generated during both the drop test and the tumble test were from the briquettes with lowest moisture level of 10%. This is probably a result of the inefficient distribution of the binder throughout the mixture.
The amount of fines generated in both tests decreased with increasing moisture level, but this trend was more pronounced with the drop test. For binder A the optimum moisture level indicated by drop test and tumble test results is 16% and 18% respectively. For binder B, a moisture level anywhere between 12% and 18% will give similar results from drop tests and tumble tests.
In tests where the binder content was varied and the moisture level was kept constant at 12%, the drop test and tumble test results followed similar trends as in Figure 3, with fewer fines being produced in the drop test. The optimum amount of binder A was found to be 0.5%. This amount produced the second lowest amounts of fines during the tumble test and drop test, at 8.8% and 32.6% respectively. From an economic point of view, adding 0.5% binder is preferable to adding 0.9%.
An amount of 1% and higher of binder B will give favourable results. Very small amounts of fines were generated during the tumble tests for 1%, 2%, and 3% binder additions. This is an indication of how well binder B briquettes can withstand an abrasive environment like transport on a conveyor belt. Hardly any fines were generated for the three higher amounts of binder additions, and hence these briquettes will stay relatively intact when tipped from a transport truck or when falling from a conveyor belt.
The optimum binder B content is therefore 1%, being the level that will ensure a low amount of fines generated.
Drying conditions
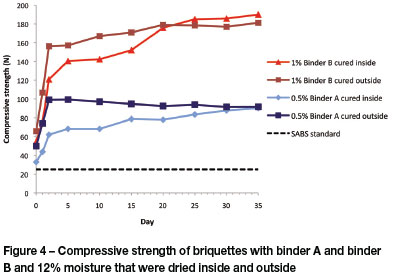
Figure 5 shows the strength gain for both binders over the first five days of drying under different conditions. Drying outside in direct sunlight exposes the briquettes to a higher average temperature than those allowed to dry indoors. This results in faster evaporation of the moisture and the different bonds created by the binders are established much earlier in the curing process. For both binders, a strength plateau is reached after five days of drying outside, and little additional strength is gained from day 5 to day 35.
For the samples dried indoors, the greatest strength gain is achieved in the first two days of curing. At this stage these briquettes do not have the same strength as the samples dried outside, but from day 5 they continue to steadily gain strength until the final strength on day 35 is similar, or close to, that of the samples that were dried outside.
Drying samples outside looks like the obvious choice as this allows for a much shorter curing process. However, it should be noted that neither of the binders results in a water-resistant briquette, and therefore when these briquettes are stored outside, they must be under cover to prevent rain damage.
Water resistance
When binder B briquettes were submerged in water, they immediately disintegrated. It was clear that these briquettes did not have any water resistance.
Binder A was expected to impart water-resistant properties to the briquettes, but these also disintegrated when submerged in water. Even after 15 days of curing the binder was not able to establish water-resistant bonds between the particles.
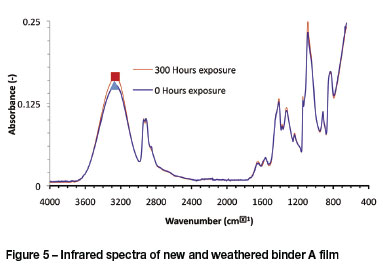
Artificial weathering of binder A
The results from attenuated total reflection (ATR) spectroscopy indicated that no weathering took place on the surface of the finder A film. In Figure 5 barely any difference is seen between the curves for the different exposure times, indicating that little to no degradation had taken place on the surface of the film after exposure of 300 hours in the QUV.
Thermogravimetric analysis
The results from TGA are plotted in Figure 6. Hardly any difference is seen between the pure coal and coal with binder mixtures. The coal, with or without binder, is thermally stable up to 400°C, and between 400°C and 600°C the binder and other volatiles decompose. Above 600°C only about 30% of the material remains, and this value is similar to the total ash content of 30.4% shown in Figure 6.
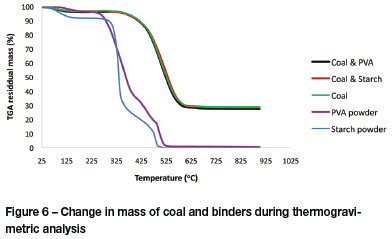
Binder A starts decomposing at about 95°C and is totally decomposed at about 530°C. Binder B shows slow decomposition from 25°C to 90°C followed by rapid decomposition between 90°C and 490°C. Binder B is totally burned off above 500°C.
The binder additions to the coal were the maximum amounts that were used throughout this study: 0.9 % binder A and 3% binder B. It is safe to say that neither of the two binders will add to the ash content of the coal as both binders totally decompose above 530°C.
Conclusions
The compressive strength of the briquettes depends on the binder addition and the moisture content. For both binder A and binder B, the optimum moisture level was 12%. The minimum binder addition for adequate strength was 0.5% for binder A, and 1% for binder B. Further additions of binder B increased briquette strength, but the higher cost of binder B renders this option uneconomical.
For binder A, the optimum moisture level was 12% to 14%. At this moisture level the largest compressive strength gains were observed, as well as a low amount of fines produced. The minimum amount of binder to be added to obtain a briquette of adequate strength was 0.5%.
For binder B the optimum moisture level was also 12%, and the minimum binder addition was found to be 1%.
The briquettes that were dried outside reached their peak compressive strength after about four days. The briquettes that dried inside took about 20 days to reach maximum strength.
Neither of the binders resulted in water-resistant briquettes, as all of the briquettes tested disintegrated when submerged in water.
ATR spectroscopy indicated that no degradation of the binder A film took place after 300 hours of exposure in the QUV.
The TGA results confirmed that neither binder A nor binder B will increase the ash content of the coal fines, as both binders totally decompose above 530°C.
The cost of binder B is higher than binder A, but its strength after 15 days of curing and the low amount of fines produced with minimum of 1% binder addition makes it the preferred binder to use.
References
Altun, N. E., Hicyilmaz, C., and Kök, M.V. 2001. Effect of different binders on the combustion properties of lignite. Part 1. Effect of thermal properties. Journal of Thermal Analysis and Calorimetry, vol. 65. pp. 787-795. [ Links ]
Dehont, F. 2006. Coal briquetting technology. http://www.almoit.com/allegati/applicazioni_particolari/15/COAL%20BRIQUETTING%20TECHNOLOGY.pdf [Accessed 13 October 2013]. [ Links ]
England, T. 2013. The economic agglomeration of fine coal for industrial and commercial use: A review of past and present work both locally and internationally. http://www.coaltech.co.za/chamber%20databases%5Ccoaltech%5CCom_DocMan.nsf/0/09EFEE68D9257C164225781B00364F21/$File/Task%204.4%201%20-%20Agglomoration%20of%20Fine%20Coal%20-%20Trevor%20England.pdf [ Links ]
ESI-AFRICA. 2014. The economic possibilities of South Africa's coal fines. http://www.esi-africa.com/the-economic-possibilities-of-south-africas-coal-fines/ [Accessed 17 June 2014]. [ Links ]
ESKOM. 2014. Coal Power. http://www.eskom.co.za/AboutElectricity/ElectricityTechnologies/Pages/Coal_Power.aspx [Accessed 17 June 2014]. [ Links ]
FEECO International. 2014. Briquettes, Granules, and Pellets - What's the difference? http://feeco.com/2012/01/11/briquettes-granules-and-pellets-whats-the-difference/ [Accessed 17 June 2014]. [ Links ]
Richards, S.R. 1990. Physical testing of fuel briquettes. Fuel Processing Technology, vol. 25. pp. 89-100. [ Links ]
SABS 1399:1999. Wood charcoal and charcoal for household use. South African Bureau of Standards. Pretoria. http://www.sabs.co.za [Accessed 20 November 2014]. [ Links ]
Sastry, K.V.S. and Feurstenau, D.W. 1977. Kinetics and process analysis of agglomeration of particulate materials. Agglomeration '77. AIME, New York. pp. 318-402. [ Links ]
Waters, P.L. 1969. Binders for fuel briquettes: a critical survey. Technical Communication 51. CSIRO. [ Links ]
Paper written on final year project work carried out in partial fulfilment of B.Eng degree in Coal Beneficiation














