Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.3 Johannesburg Mar. 2015
GENERAL PAPERS
Laser surface alloying of Al with Cu and Mo powders
S.L. PityanaI, II; S.T. CamaguIII; J. Dutta MajumdarIV
ICSIR, National Laser Centre, Pretoria, South Africa
IIDepartment of Chemical and Metallurgical Engineering, Tshwane University of Technology, Pretoria, South Africa
IIICSIR, Light Metals and Metals Processing, Pretoria, South Africa
IVMetallurgical and Materials Engineering Department, Kharagpur, India
SYNOPSIS
Laser surface alloying was used to develop copper and molybdenum aluminides by injecting premixed copper and molybdenum powder particles into a laser-generated melt pool on an aluminium substrate. Different laser processing parameters were used to produce the composite thin layers on the substrate material. The microstructure and phase constituents of the composite layer were studied by means of scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS), and X-ray diffraction (XRD) techniques. Experimental results show that the matrix structure of the metal matrix composite layer consists of θ-CuAl2 and MoAl5. Surface hardness was increased by a factor of 3.
Keywords: laser surface alloying, metal matrix composites, intermetallic phases.
Introduction
Aluminium is a very important material which is used in the automotive and aerospace industries. The choice of aluminium is based on its excellent physical properties such as low density and high specific strength. However, its application is limited by its low surface hardness and surface wear resistance, resulting in premature failure as a result of surface damage. Remedial solutions for improving surface durability in terms of wear and corrosion resistance include surface coating. Surface coating is considered to be an economical and effective process for extending the service lifetime of industrial components. In surface coating, the working surface of the substrate is coated with hard-wearing resistant particles such as carbides, borides, nitrides, oxides, and multi-component systems to form metal matrix composites (MMCs) (Wang, Lin, and Tsai, ,2003). The very distinctive feature of MMCs is that individual particles in the composite retain their properties, as well as complement each other to impart properties that cannot be found in any one of them alone (Rohatgi, Asthana, and Das, 1986; Kohara, 1990). The main goal of fabrication of Al-MMC surfaces has been to obtain improved mechanical and chemical properties while retaining the attractive properties of aluminium such as low specific weight, high specific strength, and excellent formability. The deposited coatings must be well bonded to the substrate, with no pores, micro-cracks, or splat boundaries.
Laser surface alloying techniques have received much attention as an alternative to more conventional techniques for fabricating MMCs (Dubourg, et al., 2002; Dutta Majumdar and Manna, 2011; Pityana, 2009; Aravind et al., 2004; Chong, Man, and Yue, 2001; Popoola, Pityana, and Popoola, 2011). During the laser surface alloying process the powder mixture, together with a thin surface layer of the substrate, is melted using a laser beam that is scanned across the surface. This leads to rapid solidification and formation of a coating. The rapid cooling and solidification of the laser-generated melt pool can result in the formation of various non-equilibrium phases and refined microstructures. If the powder that is injected into the melt pool contains particles which do not melt, an MMC coating is produced on the substrate surface. Due to the combination of non-equilibrium microstructures and the dispersion of hard phases, the surface properties tend to be superior to those of the untreated aluminium base material.
The current study aims at the development of an intermetallic (copper aluminide and molybdenum aluminide) dispersed composite surface on an aluminium AA1200 substrate by laser surface alloying with Cu-Mo powder mixtures. The powder composition was adjusted in order to form coatings consisting of Al-Cu and Al-Mo intermetallic phases in the laser-modified layer. Cu is added to Al alloys to increase tensile strength, hardness, and fatigue resistance, whereas Mo is added to increase corrosion resistance. The microstructure, phases, and the hardness of the coatings were investigated in detail.
Experimental procedure
Materials and coating process
The substrate material used in the study was aluminium (AA 1200) with chemical composition 0.59% Fe, 0.12% Cu, 0.13% Si, and the balance Al. Specimens with dimensions of 100x100x6 mm were laser-cut from a large sheet. The surfaces were sandblasted, rinsed, and cleaned with acetone prior to laser surface processing. Sandblasting removes the oxide layer and improves the absorption of the laser energy at the specimen surface. The Cu and Mo powder compositions and the laser processing parameters that were varied during the study are shown in Table I. For ease of reference, the samples are numbered as shown in Table I.
The energy density is defined as E = P/ (VD), where P is the laser power, V the scanning speed, and D the beam diameter.
Laser surface alloying was carried out using a Rofin Sinar DY044, CW Nd:YAG laser . An off-axis powder feeding nozzle with 2.5 mm diameter and a Precitec YW50 laser cladding head were mounted on a KUKA articulated arm robot. A 600 urn optical fibre was used to guide the laser beam to the cladding head. The powder nozzle and the laser beam were mounted 12 mm above the substrate and were arranged such that the powder stream coincided with the laser beam at the interaction zone. The mixed powders were fed into the melt pool by means of an argon gas carrier which also acted as a shield against oxidation of the melt pool. A GTV powder feeder was used to feed the powder at a rate of 2 gImin. The average particle size of the Cu and Mo powders was between 50 and 100 μm .
After laser melt injection, cross-sections of the samples were prepared for metallurgical examination. The mechanically polished surfaces were etched with Kellers's reagent (5 ml HNO3, 1.5 ml HCl, 1.0 ml HF, and 95 ml distilled H2O). The Leo 1525 scanning electron microscope, equipped with energy dispersive spectroscopy (EDS), was used for microstructure investigations. EDS was used for elemental analysis. The phases formed in the layer were identified by X-ray diffraction (XRD) using a Pan Analytical X'Pert Pro powder diffractometer with a X'Celerator detector. The radiation source used was Cu Ka (1.5402 Å). The phases were indexed using X'Pert High-score Plus software. The hardness profiles of the alloyed samples were obtained using a Matsuzawa hardness tester with a load of 100 g. Hardness profiles were constructed for each alloying process depicting the hardness from the alloyed surface down to a depth of approximately 1.6 mm.
Results and discussion
Microstructures of the alloyed surface
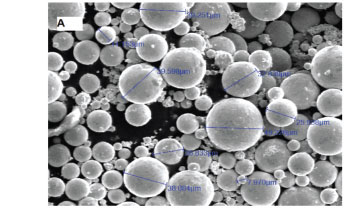
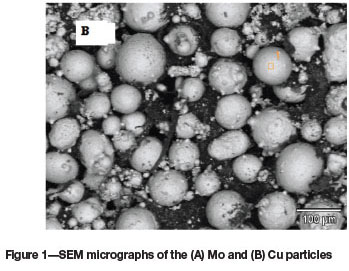
Laser surface modification was carried out by melting the Al substrate and feeding with 50%Cu-50%Mo (wt%). Figures 1 A and B show the scanning electron micrographs of the precursor Cu and Mo used in the study. The Mo particles (Figure 1A) were spherical in shape and the size distribution ranged from 5 to 50 μm. The Cu particles (Figure 1B) ranged between 5-100 μm in size and consisted of spherical and elliptical shapes.
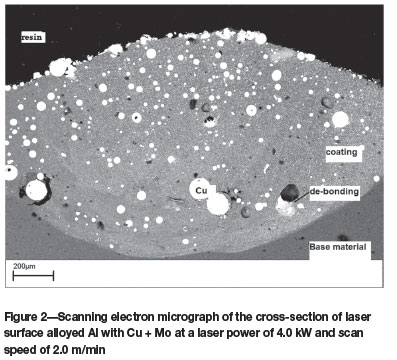
Figure 2 shows the scanning electron micrograph of the cross-section of the alloyed region perpendicular to the scanning direction. The laser process parameters used to obtain this layer were a laser power of 4.0 kW and a scan speed of 2.0 m/min. The larger unmelted particles are uniformly distributed throughout the molten zone to form a composite layer. The distribution and mixing of the particles in the solidified melt pool could be related to convective flow in the liquid melt pool. EDS analysis confirmed the presence of un-melted Cu and Mo particles. It can be observed that the alloyed layer has considerable porosity. This is due to the 'de-bonding' of the particles from the matrix.
Microstructure analysis
50% Cu-50% Mo powder mixture
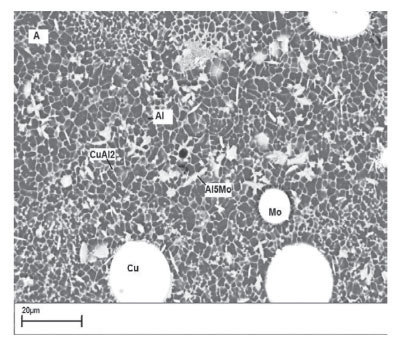
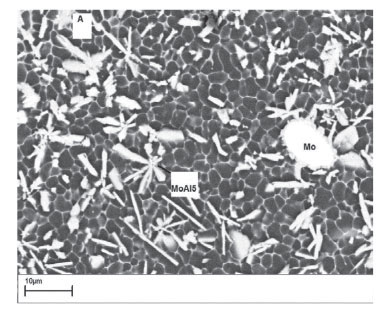
Figures 3A and 3B show the scanning electron micrographs of the laser surface-alloyed aluminium surface lased with (A) an energy density of 30 J/mm2 and interaction time of 0.12 seconds and (B) energy density of 40 J/mm2 and interaction time of 0.16 seconds. The microstructure consists of a coarse continuous network of secondary phase precipitates appearing as a light (white) areas at the dendrite boundaries enveloping the aluminium matrix phase, which appears as a grey region. Light grey, block-like structures can be observed together with un-dissolved Cu and Mo particles. The fine white eutectic network contains CuAl2 and the grey regions contain α-Al. The plate-like structures are MoAl5 intermetallics. Figure 3B shows the presence of a refined CuAl2 eutectic network. The degree of fineness is greater in Figure 3B than in Figure 3A. In addition, an increased amount of MoAl5 plate-like structures is observed in the processed layer. This can be explained in terms the higher laser energy density and longer dwell time, which increase the lifetime and the temperature of the melt pool. This allows more particles to enter the melt pool. Some particles entering the melt pool are completely dissolved, while others are retained. The melted particles react with liquid Al to form intermetallic phases of Al-Cu and Al-Mo. The Cu-Mo system is mutually immiscible in both liquid and solid states and does not form any compounds. The un-melted Cu and Mo particles form composite coatings with the Al substrate.
25%Cu-75% Mo powder mixture
Figures 4A and 4B show the scanning electron micrographs of the top surface of laser surface alloyed aluminium treated with (A) an energy density of 30 J/mm2, and (B) an energy density of 40 J/mm2. The variation in the microstructure of the alloyed layer can be explained on the basis of the laser absorption coefficients of the Cu and Mo particles. The Mo powder absorbs more laser power, resulting in a higher melt pool temperature which leads to the melting of the Cu powder. However, the high volume fraction of the Mo particles in the powder mixture and their high melting temperature (2620°C) ensures the retention of a significant number of Mo particles in the alloyed layer. Due to the low melting temperature of Cu (1083°C), most Cu particles dissolve to form the e-Al2Cu intermetallics. In both cases, the matrix consists of a eutectic mixture of alternate layers of Al2Cu and α-Al inside the dendritic regions. Figure 4A shows the Al-Mo intermetallic phases existing in different morphologies. The Al-Mo dendrites have flower-like and long needle-like appearances. Figure 4B shows the Al-Mo dendrites as needle-like or plate-like morphologies. This expected variation is due to the incident energy and longer dwell time of the laser beam on the substrate, which lead to high heating and cooling rates. Qiu, Almeida, and Vilar (1998) reported that the MoAl5 intermetallic can be observed in different allotropic forms, depending on the heating and cooling rates.
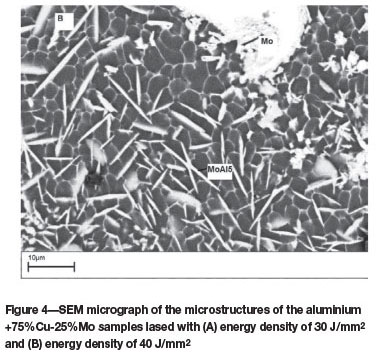
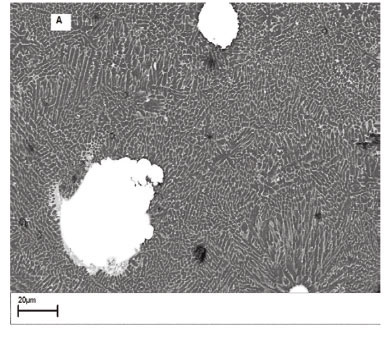
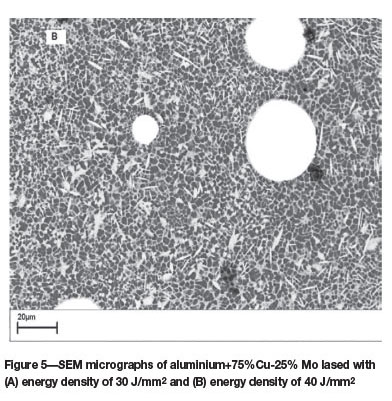
75% Cu-25% Mo powder mixtures
Figures 5A and 5B show the scanning electron micrographs of the top surface of laser surface alloyed aluminium lased with (A) an energy density of 30 J/mm2, and (B) energy density of 40 J/mm2. The microstructure (Figure 5A) consists of a continuous B-Al2Cu eutectic network engulfing the α-Al phase. Unmelted Mo particles are evident in the micro-structure. The Mo-Al intermetallic phases can be distinguished by their block-like appearance. At an energy density of 40 J/mm2, the Cu particles were completely melted and reacted with Al to form very fine cellular and columnar dendritic structures (Figure 5B). The partially melted Mo particles are randomly distributed in the alloyed zone. Owing to the low mutual solubility of Mo and Cu, no Mo-Cu intermetallic phases were observed in the alloyed layer.
XRD analysis
Figure 6 shows the XRD profiles of the alloyed zone developed on the surface of Al+50%Cu-50% Mo. The dominant XRD reflection peaks are due to the Al substrate at 2θ = 38.47° (111), 44.72° (200), 65.1° (220), 78.23°, (311), and 82.44° (222). The in situ synthesized θ-CuAl2 intermetallic has XRD peaks at 2θ = 42.7° (112) and 73.64°. The Al5Mo intermetallics reflection peaks are found at 40.47° and 58.59°. No Cu-Mo phases were detected in the laser processed layer, due to limited solubility of the Cu and Mo. The XRD peak positions do not show any significant difference in the structural compositions between the layers formed using 30 and 40 J/mm2 laser energy densities.
Figure 7 shows the XRD profiles of the alloyed zone developed on the surfaces alloyed with 25%Cu+75%Mo at energy densities of 30 and 40 J/mm2. It is evident that the coatings contain the Al, MoAl5, and Al2Cu intermetallic phases. SEM micrographs of the layers showed unmelted particles of Cu and Mo distributed homogeneously throughout the laser deposited layer.
Figure 8 shows the XRD profiles of the alloyed zone developed on the surfaces of samples 649 and 650 (alloyed with 75%Cu-25%Mo). Sample 649 shows additional peaks for the 9-Al2Cu intermetallic detected at 2θ = 20.62° (110), 29.63° (200), and 47.25° (310). The results also show the possible formation of Cu9Al4 at reflection angles of 26.63° and 49.56°. The MoAl5 intermetallics are detected in the alloyed surface. In the case of sample 650, the lower angle (20-35°) diffraction peaks corresponding to Al2Cu are not detected; however, the peak at 47.25° was detected. From these results it is evident that dominant phases synthesized in the alloyed layer are the Al2Cu and MoAl5 intermetallics.
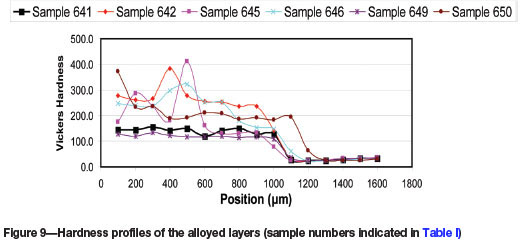
Hardness
Hardness measurements were carried out using a Vickers micro-hardness tester at an applied load of 100 g. Hardness measurements were made across the depth of the laser treated surface. Figure 9 shows the Vickers micro-hardness profiles of the samples. The alloyed zone extends up to 1000 'm into the substrate. The hardness of the alloyed zone is much higher (approx. 100-250 HV) than that of the Al substrate (approx. 25 HV). In all the alloyed samples, the hardness is always higher for samples treated at the higher energy density, regardless of the powder composition. The highest hardness was obtained for samples alloyed with 50%Cu+50%Mo at 40 J/mm2 (approx. 280 HV), 25%Cu+75% Mo at 40 J/mm2 (approx. 250 HV), and 75%Cu+25% Mo at 40 J/mm2 (approx. 200HV). The increase in hardness is attributed to the presence of the CuAl2 and MoAl5 intermetallic phases in the alloyed layer. The microstructures of these samples showed a much-refined θ-CuAl2 eutectic and a higher volume fraction of MoAl5 intermetallic phases; these factors are related to the increased hardness values obtained.
Conclusions
1. An Al-Cu-Mo composite was successfully synthesized by the laser melt injection method. The composite layer consisted of the CuMo reinforcements and Al-Cu and Al-Mo intermetallic phases
2. XRD analysis of the layers showed that the main phases are θ-CuΑ2 and MoAl5. The Cu9Al4 phase was obtained with an increased Cu content in the mixture at higher laser scan speed
3. The Cu-Mo compounds were not formed in the alloyed layers, as shown by the XRD analysis
4. The microstructures of the processed zones are refined, cellular, and columnar according to the laser processing parameters and cooling rates
5. The composite coatings have much higher hardness than the Al substrate.
Acknowledgements
The authors would like to thank the CSIR National Laser Centre for financial support, and Mr Lucas Mokwena for helping with the experiments 5.5
References
Wang, S.W., Lin, Y.C., and Tsai, Y.Y. 2003. The effects of various ceramic-metal on wear performance of clad layer. Journal of Materials Processing Technology, vol. 140. pp. 682-687. [ Links ]
Rohatgi, P.K., Asthana, R., and Das, S. 1986. Solidification, structure and properties of cast metal-ceramic particle composites, International Materials Reviews ,vol. 31. pp. 115-139. [ Links ]
Kohara, S. 1990. Fabrication of SiCp-Al composite materials. Materials and Manufacturing Processes, vol. 5, no. 1. pp. 51-62. [ Links ]
Dubourg, L., Pelletier, H., Vaissiere, D., Hlawka, F., and Cornet, A. 2002. Mechanical characterisation of laser surface alloyed aluminium-copper systems. Wear, vol. 253. pp. 1077-1085. [ Links ]
Dutta Majumdar, J. and Manna, I. 2011. Laser material processing. International Materials Reviews, vol. 56, no. 5-6. pp. 341-388. [ Links ]
Pityana, S. 2009. Hardfacing of aluminium by means of metal matrix composites produced by laser surface alloying. 5th International WLT-Conference on Lasers in Manufacturing, Munich, Germany, 15-18 June 2009. pp. 439-444. [ Links ]
Aravind, M., Yu, P., Yau, M.Y., and Ng, D.H.L. 2004. Formation of Al2Cu and AlCu intermetallics in Al(Cu) alloy matrix composites by reaction sintering. Materials Science and Engineering A, vol. 380. pp. 384-393. [ Links ]
Chong, P.H , Man, H.C., and Yue, T.M. 2001. Microstructure and wear properties of laser surface cladded Mo-WC MMC on AA6061 aluminium alloy. Surface and Coatings Technology, vol. 145. pp. 51-59. [ Links ]
Popoola, A.P.I. Pityana, S. L., and Popoola, O.M. 2011. Laser deposition of (Cu+Mo) alloying reinforcements on AA1200 substrate for corrosion improvement. International Journal of Electrochemical Science, vol. 6. pp. 5038-5051. [ Links ]
Qiu, Y.Y., Almeida, A., and Vilar, R. 1998. Structure characterisation of a laser-processed Al-Mo alloy. Journal of Materials Science, vol. 33. pp. 2639-2651. [ Links ]
Paper received May 2013.
Revised paper received Oct. 2014.