Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.2 Johannesburg Feb. 2015
GENERAL PAPERS
The behaviour of free gold particles in a simulated flash flotation environment
T.D.H. McGrathI; J.J. EksteenI; J. HeathII
IDepartment of Metallurgical Engineering and Mining Engineering, Western Australian School of Mines, Curtin University, Australia
IIOutotec South East Asia Pacific, West Perth, Australia
SYNOPSIS
A reliable laboratory method to characterize the response of free gold particles to flash flotation conditions has been developed. The test has been performed on free milling gold ores as well as synthetic ores, using either a gravity concentrate or gold powder as the gold source, to assess the floatability of gold particles. Trends in free gold flotation kinetics, as well as size and milling effects, were identified for gold recovery based on the different feed types, reagent dosages, and residence times. It was shown that the ultimate recoveries and kinetic trends of gold particles from the gravity concentrate could be enhanced with increased dosage of collector, potassium amyl xanthate. Interestingly, in comparison to gravity-recoverable gold, recovery from pure Au powders was better in collectorless flotation, and cumulative recovery decreased with higher levels of collector addition. Improved coarse particle recovery appeared linked to increased collector additions for both the gravity concentrate and the pure gold powders. In general, coarse gold particles demonstrated slower kinetic rates thaen the fine or intermediate components in comparable tests.
Keywords: gold, flotation, flash flotation, natural hydrophobicity, kinetics.
Introduction
The behaviour of free gold in flash flotation is currently poorly understood (Dunne 2005), especially when in competition with a gravity recovery unit in a closed-loop milling circuit, although an overlap has been identified in which both units can recover particles between 212 μm and 38 μm. This research aims to identify parameters that may determine whether free gold particles will be recovered by either unit in this competitive size range. Identifying the impact of variables such as mineralogy, reagents, mechanical factors, and physical characteristics (such as size, shape, surface area, elemental composition, etc.) on floatability will enable optimization of combined gravity and flash flotation circuits. This paper, the second in a series, is focused on the comparison of free gold and pure gold powder recoveries in laboratory flotation tests as a function of collector (potassium amyl xanthate, or PAX) addition. The first paper (McGrath et al., 2013) established the method used to study the behaviour and characterize the ultimate content of free gold recoverable by flash flotation. The knowledge gained from this research contributes to a better understanding of the impact of particle size, milling effects, residence time, and collector additions upon the recovery of free gold in the milling circuit.
Background
Several plants use batch centrifugal concentrators (BCCs) and flash flotation unit operations in a closed-loop milling circuit as an option for processing complex ores containing free gold as well as gold locked in a sulphide matrix. BCC circuits are used to recover the larger particles of free gold, roughly +106 μm, while a flash flotation circuit produces a sulphide concentrate encompassing smaller free gold particles (-106 μm) and gold contained in sulphides. Based on plant surveys undertaken by the Curtin University Gold Technology Group (2008), the two units will tend to compete for particles in the -212 +38 μm range, as shown in Figure 1.
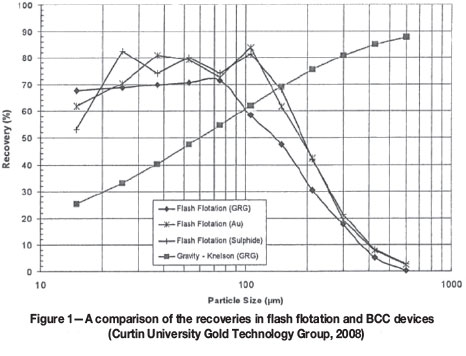
Because knowledge of the behaviour of free gold recovery in a closed-loop milling circuit with parallel flash flotation and gravity recovery units is limited, an improved understanding of the behaviour of gold in this situation will provide greater confidence in the application of such processes to the processing of complex gold ores.
Gravity concentration
Gravity-recoverable gold (GRG) is a specific term that refers to free gold reporting to the concentrate stream with a small mass yield if separations are performed using BCCs. GRG is comprised of primarily of 'free gold', because the BCC units are not designed to recover bulk sulphide material. The 'GRG test' (Laplante and Dunne, 2002) and model generates results that can be used to model the amenability of an ore to gravity recovery by BCC in a milling circuit closed by a hydrocyclone. The GRG model developed by Laplante can als be used to estimate flash recovery (Laplante and Staunton, 2005; Laplante and Dunne, 2002). This is because the GRG test defines an ore characteristic, not BCC machine characteristics. Therefore it determines the overall gravity recovery potential of an ore and the maximum amount of GRG per sizeclass that can be recovered. Both the GRG test and laboratory/plant-based models are available for use.
Flash flotation
The SkimAir flash flotation cell was developed by Outokumpu (now Outotec) in the early 1980s to 'flash off fast-floating liberated minerals of high value (Coleman, 2010). It was designed to be used ahead of conventional flotation in the circulating load of a mill in order to reduce overgrinding of sulphides (Bourke, 2002). Teague et al. (1999) have shown that flotation of free gold is affected by physical constraints such as shape and size of particles, degree of water and gangue transport to the froth, stability of the froth, and extent of sulphide bubble loading, which provides a barrier t hydrophobic bubble attachment of free gold. It has been suggested that fine gold particles are strongly hydrophobic and good candidates for flash flotation (Laplante and Dunne, 2002). Unfortunately, the effect of an industrial flash flotation cell on the recovery of free gold in Australia has been difficult to determine, as flash flotation has been incorporated at the design stage and there is little plant data available on free gold recovery before and after the introduction of the unit (MacKinnon et al., 2003). To date, Laplante's GRG test is the only method available to approximate the expected recovery of free gold in a flash flotation cell.
The gravity/flash flotation relationship for GRG
When both gravity concentration and flash flotation are employed in a milling circuit, flash flotation can be used in parallel, series, or cleaning arrangements with BCC units, as seen in Figure 2. In a cleaning application, the flash flotation cell creates a sulphide concentrate which is then secondarily treated by gravity recovery with removal of GRG from the bulk sulphide concentrate. In series, the BCC treats a portion of the flash tails, while in parallel, the flash and gravity units share the same feed, usually the cyclone underflow, and the tails streams are returned to the milling circuit to close the loop. In the parallel arrangement, the nature of the particles recovered to each unit and the factors affecting recovery of the GRG are not completely understood, due to the interaction of many complex factors.
In general, when the GRG content is high and the size fraction is coarse, free gold is easy to recover and concentrate in gravity operations. BCC units operate ideally to concentrate coarse free gold particles larger than 106 μm. Some particles between 106 and 38 μm are recovered, but recovery is likely to depend on particle shape. BCC systems give poor recoveries of gold particles smaller than 38 μm (Laplante and Staunton, 2005). Despite the ideal particle size-gravity gold recovery curve presented for BCCs in Figure 1, Wardell-Johnson et al. (2013) have shown that the size-by-size recovery of GRG is far from an ideal monotonic function, with actual BCC devices often showing a U-shaped curve (rather than a monotonically increasing S-shape) for intermediate particle sizes. Further understanding of the factors impacting the behaviour of GRG in this type of competitive, yet complementary, parallel operation is the primary subject of this research.
Natural hydrophobicity of gold and the impact of collector (PAX)
Natural hydrophobicity
Tennyson (1980) demonstrated that pure metallic gold is hydrophilic. However, fine free gold will float better than gangue material without the addition of collector, and O'Connor and Dunne (1994) have also shown that untarnished gold of the appropriate size can be readily floated with only a frother in a process referred to as 'collectorless flotation'. This hydrophobic behaviour often displayed by gold particles is due to a high Hamaker constant (Drzymala, 1994), indicative of Van der Waal's interactions resulting in a strong dispersive attraction for water (Dunne, 2005). The floatability of gold is known to be enhanced by surface coatings of some organic compounds and silver content (either as rimming or in the form of electrum), while calcium ions and some forms of sulphur can act as depressants. It has been suggested that when native gold surfaces are hydrophobic due to contamination by organics in nature, fine gold particles may be harder to recover by flotation (Aksoy and Yarar, 1989).
Collector
Xanthates, a group of anionic collectors based on bivalent sulphur, are used in flotation plants (together with other collectors) to enhance gold recoveries (O'Connor and Dunne, 1994; Wills, 2006). PAX was the collector chosen for this investigation because it is readily available and often used in laboratory flotation test work, so comparisons may be made between these results and data produced in other studies. Furthermore, free gold floats well in the presence of xanthate collectors, but not if the particle size is too large, or if calcium salts or minerals, or Na2S are present (Teague et al., 1999). Not only is gold hydrophobicity enhanced by the addition of collectors such as xanthates, dithiophosphates, and dithio-phosphinates, but untarnished gold requires even less addition of these collectors than tarnished gold to become suitably hydrophobic (Chryssoulis and Dimov, 2004). Secondary collectors, or promoters, can further increase recovery, with dithiophosphates being the most widely used promoters in gold flotation (O'Connor and Dunne, 1994). As with tarnished particles, higher collector additions may be required to float coarser particles, as demonstrated in this study. However, studies with quartz have shown that extra collector added to float coarse or tarnished material may instead be consumed by fine particles with large surface areas (Vieira and Peres, 2007).
Physical parameters
Comminution
As mentioned by Dunne (2005), the impact of milling on the floatability of free gold particles has been an issue of debate. It was suggested by Taggart (1945) and Pevzner et al. (1966) that milling may decrease a gold particle's ability to be recovered by flotation because of the impregnation of gangue material. Pevzner et al. (1966) also proposed that passivation of the gold surface during milling would lead to reduced flotation recoveries. Conversely, Allan and Woodcock (2001) hypothesized that work-hardening of gold particles during milling could activate the surfaces and improve floatability. Work-hardening will strengthen the surface of a metal by plastic deformation and can change the surface finish, thus potentially affecting the adsorption of collector. Silver content is expected to promote the flotation of GRG particles as compared to pure Au powders, and this must also be considered as the GRG concentrate particles contain between 5-20% silver (roughly 10% on average). This is because silver floats preferentially to gold in the presence of xanthates.
Particle size
Particle size has a strong influence on flash flotation and BCC recovery for several reasons. Firstly, liberation is directly related to particle size and flotation will proceed only when a particle is sufficiently liberated (Zheng et al., 2010). Chalcopyrite flotation studies have shown that a coarse liberated particle floats similarly to an intermediate partially liberated particle, and a coarse particle will float slower than an intermediate particle of similar composition (Newcombe et al., 2012; Sutherland, 1989). Secondly, reagent additions and pH control have a greater influence on coarse particle flotation than other sizes (Trahar, 1981). Recovery is maximized in the 100-10 μm size fraction and drops off significantly above and below that range, with few particles greater than 300 μm able to be floated (Trahar, 1981). The literature suggests that free gold of +200 μm cannot be floated effectively (Malhotra and Harris, 1999). In most flotation plants, sulphide particles larger than 150 μm are considered too large for conventional flotation, although it is a widely held misconception in industry that flash flotation can and will recover even larger particles (Newcombe et al., 2012). It has also been shown that BCCs recover just 40% of +38 μm gold particles and only 10% of -38 μm gold particles (Chryssoulis and Dimov, 2004). With this knowledge, the size ranges of interest in this study are +212 μm, -212 + 38 μm, and -38 μm.
Surveys suggest that free gold particles larger than 212 μm will preferentially report to a gravity concentrator when gravity and flash flotation are operated in a closed-loop milling circuit, while -38 μm fractions of free gold particles are usually captured in the flash flotation concentrate of the same circuit. Particles in the size fraction -212 +38 are of special interest, as this is the zone of competition between the gravity and flash units, and further understanding of the response of free gold particles in this intermediate range to varying flotation conditions can be applied to optimize recovery in industrial applications. For ease of discussion, the +212, -212 +38 (or just +38), and -38 μm size fractions in the test work are referred to as coarse, intermediate, and fine, respectively throughout the remainder of this paper. Particle shape, surface area, organic and mineral coatings, and elemental rimming are also important in determining the floatability of free gold and will be addressed in a subsequent paper by the authors.
Experimental method
The aim of this study is to compare trends in flotation kinetics for particles of varying size and nature as affected by the addition or absence of a collector. Gold from two sources was floated according to the free gold flash flotation test developed by McGrath et al. (2013), with PAX addition and gold type being the only variables. As noted in the results, the superficial gas velocity (volumetric gas flow per unit cross-sectional area of vessel) remained constant at 0.0074 m/s for all tests.
Two synthetic ores were created for the laboratory flotation test work. One contained a BCC gravity concentrate (P100 of 600 μm), created by blending multiple concentrates, from primarily Australian sources, split into 5 g subsamples yielding head grades of about 13-16 g/t when added to silica (P100 of 600 μm, with a size distribution similar to flash feed material). A bulk assay of the BCC concentrate established that the concentrate had a gold-to-silver ratio of 9:1, although scanning electron microscopy (SEM) demonstrated that individual gold particles vary greatly in the ratio and pattern of Ag placement in the particle. Images of particles typically found in the GRG concentrate are shown in Figure 3. The second synthetic ore was created using the same silica blend as the first, but used synthesized pure gold powders (P100 2 50 μm, supplied by Sigma Aldrich) to obtain a head grade of 30-40 g/t. Images of typical gold powder particles can be found in Figure 4.


The laboratory tests were repeated on 1 kg charges six times for each of the six conditions in order to produce enough combined concentrate mass to be screened into the three size fractions of interest. The replicate tests ensured that average masses and concentrations reported were statistically representative. Previous comparable test work demonstrates that strict adherence to the methods detailed in the standard operating procedure (SOP) yields average standard deviations of ±0.45% for mass pull and ±4.63% for gold recovery (McGrath et al., 2013). Each set of six conditions produced seven concentrates and a tails sample, all of which were screened into three size fractions (+212, +38, and -38 μm), yielding 21 samples per test or 147 for the entire data-set. Concentrate samples were fire-assayed to extinction while splits of the tails samples were subject to both fire assay and intensive cyanide leach by rolling bottle in order to better close the mass balances. Because the mass of gold in the test was either known, in the case of the powders, or calculated in the case of the concentrate, inconsistencies in the gold and mass balances are attributed to the nugget effect in the coarse, and to some extent, the intermediate tails samples. The nugget effect compromises the ability to achieve representative grade or concentration results due to non-uniform distribution of gold in the assayed sample as compared to the bulk material. The impact of the nugget effect is most noticeable in precious metal assays of coarse size fractions and small sample size, yielding erroneously high or low values. Previous work on this GRG concentrate has shown that the contained gold is easily leached, so cyanidation was conducted on larger splits of sized tail samples for more accurate assay values.
Brezani and Zelenak (2010) describe flotation as a process that is affected by many properties, not just physico-chemical and surface properties but many other chemical and mechanical factors. It is because of this complexity that flotation is often described as a simplified first-order kinetic phenomenon (Kelly and Spottiswood, 1989). Kinetic rates have been calculated for each size fraction in all data-sets of this study using the Kelsall approach, as presented in Equation [1]. Although this method was developed many years ago (Kelsall, 1961), its continued relavance has been evaluated by various authors (Kelly and Spottiswood, 1989; Kelebek and Nanthakumar, 2007; Brezani and Zelenak, 2010) throughout the years and it was recently applied to evaluate the kinetics of sulphides in flash flotation (Newcombe et al., 2012a).

where:
Co = the original concentration in the pulp
C = the concentration in the pulp at time t
t = elapsed time in the duration of the experiment (min)
kf = rate constant for fast-floating material (min-1)
ks= rate constant for slow-floating material (min-1)
α, β, and γ = coefficients used to fit data for non-floating (Ø), slow-floating (s), and fast-floating (f) material, the sum of which equals unity.
Results and discussion
The recoveries of particles in individual size fractions are important because they demonstrate which proportion of the material is recovered under specific conditions; this information is termed 'fractional recovery' in this paper. For example, 14% of coarse gold from the GRG concentrate was recovered without PAX addition, as shown in Figure 5, suggesting that 86% of the coarse GRG concentrate gold reported to tails in this test. Cumulative recovery refers the total or overall recovery, which is the sum of the recoveries for all size fractions; the cumulative recoveries are given at the end of this section. For reference, industrial flash flotation processes usually operate with around 10-25 g/t of PAX addition, with about two or three minutes' residence time. The first two minutes of laboratory testing (which have been represented by the initial four data points in Figures 5-12 and which includes all data presented in Figure 14), roughly represent flash flotation, and have been denoted as the 'flash period'.
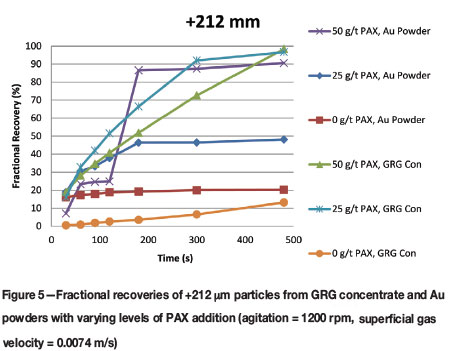


The floatability of coarse, +212 μm, gold is of interest in this study because this is the size fraction generally considered to be too large for flotation, with preference given to gravity recovery in this size fraction. Figure 5 demonstrates that:
➤ Coarse gold recovery and flotation kinetics were improved with the addition of PAX, although flash period recoveries are less than the ultimate recoveries. In this data-set the GRG recoveries are superior compared to the Au powders in tests involving PAX
➤ Using the free gold flash flotation test, 97% of the coarse free gold contained in the GRG concentrate was recovered with either 25 or 50 g/t PAX addition. While the coarse Au powder particles were 90% recovered with 50 g/t PAX, only 48% recovery was achieved when the same particles were floated with 25 g/t PAX. This can be attributed PAX being available in excess of the concentration required to create the necessary monolayer on the surface of gold. The surplus PAX forms additional layers and the non-polar ends are either concealed or oriented away from the water, which effectively reduces hydrophobicity
➤ Collectorless flotation recovered less coarse free gold than tests with PAX, resulting in recovery of only 20% of Au powder and 13% of free gold from the GRG concentrate.
The intermediate, -212 +38 μm, size range is of particular interest because this is the suggested area of competition between BCCs and flash flotation in parallel operation. A review of the data in Figure 6 suggests that:
➤ A moderate PAX addition improved intermediate particle recovery, although recoveries of both the GRG and the Au powders were slightly better with the lower level of PAX addition
➤ A much lower ultimate recovery was achieved for intermediate GRG material as compared to the coarse particle GRG at both levels of PAX addition. As with the coarse particle data, the intermediate Au powder particle recovery was lower than comparable GRG recoveries
➤ Collectorless flotation recovered the least amount of intermediate particles from both the Au powder and GRG concentrate, at less than 15% and just over 0%, respectively.
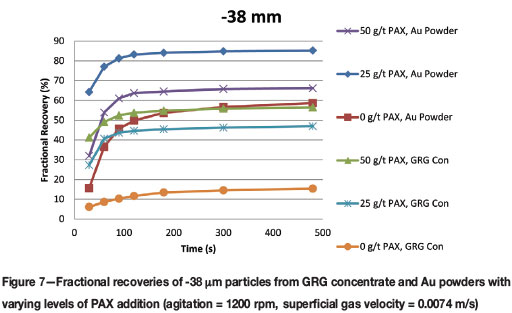
The recoveries of -38 μm particles can be found in Figure 7. Interestingly, this is the only size range where the recoveries of Au powder particles were better than from the GRG concentrate. This is also the size range for which flash flotation is recommended for free gold recovery within the milling circuit.
➤ The fine Au powder demonstrated the highest ultimate recovery with 25 g/t PAX addition. Recovery decreased when PAX was increased to 50 g/t. Recoveries with collectorless flotation of Au powders were the same as with 50 g/t PAX addition to the GRG concentrate, with respective recoveries of 59% and 57% not being statistically different
➤ Again, the fine GRG particles recovered in collectorless flotation showed the poorest recovery, just as with the coarse and intermediate size fractions.
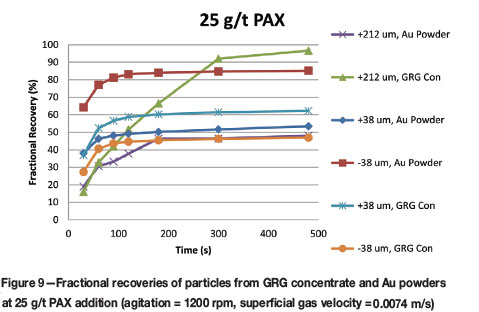
Figures 8-10 allow comparison of recovery trends grouped by level of PAX addition. The recoveries of gold with 25 g/t PAX addition, given in Figure 9, are of particular interest because this level of collector is most similar to that in industrial flash flotation. In particular, opposing trends of improved recovery for some particle types with 25 g/t PAX addition are noted. For example, GRG yielded better recoveries with increasing particle size, while decreasing Au powder particle size improved recovery. Here, the highest ultimate recoveries are from the coarse fraction of GRG concentrate and the fine Au powders.
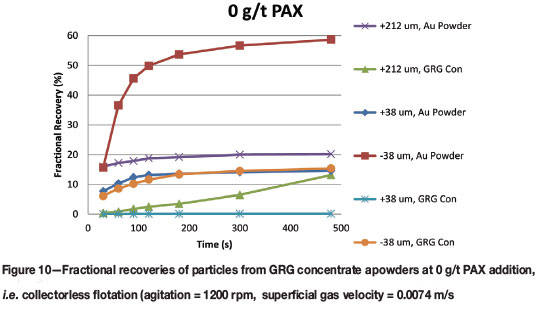
Collectorless flotation results, presented in Figure 10, are important because they reveal the differences in the inherent floatability of the two types of particle under the given conditions. Without collector, recoveries from the fine Au powders were better than from all other particle size and type combinations, with reduced recoveries for the intermediate and coarse fractions. A similar trend is noticed with the GRG concentrate, where fine particle recoveries are better than the intermediate (which shows no appreciable recovery) or coarse particles.
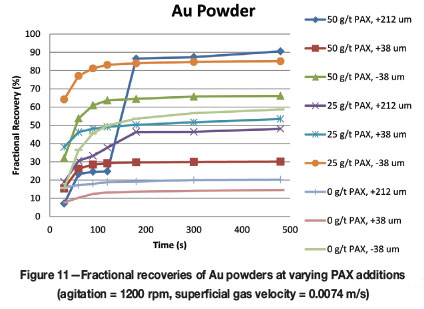
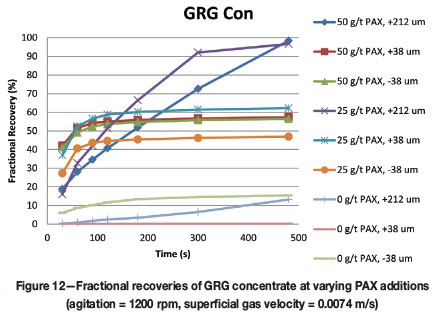
The effect of varying PAX additions for the two types of gold particles is given in Figures 11 and 12. This information is of interest as it gives baseline theoretical recoveries for unmilled, pure gold particles displaying various shapes in the size ranges of interest and can be used to demonstrate the influence of milling, silver content, and surface effects on the ability of PAX to float the free gold. In this test work:
➤ Fine Au powder particle recoveries were mostly better than the larger sizes, regardless of PAX addition, especially in the case of collectorless flotation. Fine and intermediate particle recovery decreased with 50 g/t PAX addition, which is probably a result of overdosing of the collector and, as a consequence, reduced hydrophobicity
➤ Despite slow kinetics, the coarse free gold from the GRG concentrate reached the highest recoveries at PAX additions of 50 g/t and 25 g/t .
➤ The fine and intermediate GRG particles showed intermediate recoveries at both the 50 g/t and the 25 g/t PAX additions. Interestingly, recoveries of intermediate particles were similar at either PAX addition level. The fine particles exhibit increased recovery with further PAX additions. This may indicate that over-ground particles have a larger surface area and require more collector to achieve maximum recovery in flotation; or that comminution has a deleterious effect on the surface of the fine particles, decreasing the capacity to adsorb collector
➤ While all GRG particles achieved poor recoveries in the flash period without the addition of PAX, the intermediate GRG concentrate was not recovered at all without the addition of PAX. However, with PAX additions, recoveries of intermediate and fine GRG particles were better than the coarse particles.
The cumulative recoveries of each particle size for each test condition are shown in Figure 13. Although this figure does not contain any kinetic information, this is the first time that cumulative recoveries for the laboratory flash flotation test have been documented. Previous test work (McGrath et al., 2013) has shown that differences of more than 4.6% in total gold recovery can be deemed statistically significant when using the flash flotation laboratory test for free gold. When data is reported in terms of cumulative recovery, as in Figure 13, a few trends are evident, some of which are only a function of the particle size distribution and do not offer any suggestions about floatability.

➤ Firstly, the coarse particles float better with increasing PAX, particularly in the case of the GRG concentrate
➤ Secondly, recoveries from the intermediate Au powder are better than from the intermediate GRG concentrate by collectorless flotation; while the intermediate fractions of both powder and concentrate contribute similarly to cumulative recovery in the 25 g/t PAX trial
➤ Thirdly, the cumulative recovery from the Au powders is maximized at 25 g/t PAX, decreasing when PAX is increased to 50 g/t. Conversely, the highest cumulative recovery from GRG is obtained at the highest level tested for PAX (50 g/t), and the combined recovery drops when collector dosage is lowered to 25 g/t.
Figure 14 shows ideal cumulative recoveries for the flash flotation period during the first two minutes (four data points) of the laboratory testing, as this is of specific interest in this study. Interestingly, the trend of increased recovery of fine GRG with increased residence time is evident when the flash period flotation data in Figure 14 is compared to the cumulative recoveries shown in Figure 13. Yet, this trend was not so evident for the coarse and intermediate GRG or any of the Au powder particles.

Kinetic values presented in Table I have been calculated using the method of least squares to fit the coefficients in Equation [1] and the Solver function in Microsoft Excel to solve for unknowns. A mean absolute percentage error (MAPE) has also been calculated as a measure of accuracy for each data-set; this is a common method for determining forecast error in timed data series. The α, β, and γ coefficients are useful in comparing the amount of material recovered in each data-set. Analysis of these coefficients and the kinetic rate data reveals that:
➤ A majority of the floatable intermediate and fine Au powder and GRG concentrate particles were recovered with the addition of PAX in the fast-floating portion, while the remainder of the gold particles contained in the pulp reported to tails, leaving hardly any material in the slow-floating category
➤ The lack of a slow-floating portion was more pronounced in the Au powders than in the GRG concentrate
➤ The fast-floating portion of GRG in the fine fraction increased with increased PAX addition and the non-floating component decreased; however, despite changes in PAX levels the slow-floating portion of GRG in the fine fraction remained similar
➤ The coarse fraction of GRG particles had a large proportion of slow-floating material, which was also not decreased by the addition of PAX
➤ It is important to note the high MAPE values, like those seen in the +212 μm data, show poor fit for estimation of kinetic values, which is probably due to sampling errors consistent with the nugget effect.
Conclusions
The recovery of GRG concentrate was enhanced with increased additions of PAX, as clearly seen in the cumulative recovery data. The recoveries from Au powders were better by collectorless flotation than from the GRG concentrate but, unlike the GRG concentrate, cumulative recovery of fine and intermediate particles decreased with higher levels of PAX addition, probably due to collector overdosage.
Interestingly, higher recovery of coarse particles appears to be directly linked to higher additions of PAX, for both GRG concentrate and the Au powders. Without large dosages of PAX the coarse GRG particles had slow kinetic rates, suggesting they are unlikely targets for recovery in industrial flash flotation. Unfortunately, assays for the coarse particles were skewed by problems related to the nugget effect. Therefore, absolute recovery values may shift if sufficient sample mass can be assayed to counteract the nugget effect; however, the recovery trends are expected to be similar. Recoveries of the intermediate Au powder and GRG concentrate particles were similar in the laboratory flotation test with PAX collector. Because the Au powders displayed superior potential for collectorless flotation in this size range (as well as the other two size ranges) compared to the GRG concentrate, it is suggested that milling could have a damaging effect on the natural hydrophobicity of free gold. Recoveries of fine Au powder particles were better than the fine GRG in all experiments. This is unexpected, because the literature suggests that flotation kinetics are proportional to the silver content of the GRG. Therefore, the decrease in kinetics and recovery for fine GRG particles is possibly further evidence of the deleterious effect of milling on the floatability of GRG particles.
Kinetic evaluations indicate that intermediate and, to some extent, fine gold particles from both sources were either recovered in the first 30 seconds or reported to tails. The kinetic data, coupled with flash flotation period recoveries comparable to plant flash recoveries (with an addition of PAX), suggests that both intermediate and fine GRG particles are appropriate targets for industrial flash flotation, though cumulative recoveries are low.
Further work is being conducted on samples obtained during plant surveys to help development of a size-dependent flotation model for the recovery of GRG concentrate to flash flotation or gravity recovery when these units are used in a closed-loop milling circuit. Additionally, this work focuses on identifying variations in physical parameters (shape, composition, surface area, and roughness) of gold particles found in concentrate samples as determined by QEMSCAN and Micro CT. The information gained will lead to improved understanding of the recovery of free gold in parallel flash flotation and gravity operations.
Acknowledgments
The authors wish to thank the AMIRA P420E sponsors (AngloGold Ashanti, Australian Gold Reagents, Barrick Gold, Gekko Systems, Harmony, Kemix, Magotteaux, Newcrest Mining, Newmont, Norton Gold Fields, Orica Australia, Rangold, St Barbara, and Tenova) for financial and technical support, as well as their patience, expertise, and valuable input in revising this paper.
References
Aksoy, B S. and Yarar, B. 1989. Natural hydrophobicity of native gold flakes and their flotation under different conditions. Processing of Complex Ores. Dobby, G.S. and Rao, S.R. (eds.). Pergamon Press, Halifax. pp. 19-27. [ Links ]
Allan, G.C. and Woodcock, J.T. 2001. A review of the flotation of native gold and electrum. Minerals Engineering, vol. 14, no. 9. pp. 931-961. [ Links ]
Brezani, I. and Zelenak, F. 2010. MATLAB tool for determining first order flotation kinetic constants, Kosice, Slovakia. http://www.mathworks.com.au/matlabcentral/fileexchange/28583-flotation-kinetics-equation-fitting/content/html/Flotation_fitting.html [Accessed 24 March 2014]. [ Links ]
Bourke, P. 2002. Flash flotation of copper and gold. Output, vol. 3. pp. 7-11. [ Links ]
Chryssoulis, S. L. and Dimov, S.S. 2004. Optimized conditions for selective gold flotation by TOF-SIMS and TOF-LIMS. Applied Surface Science, vol. 231-232. pp. 265-268. [ Links ]
Coleman, R. 2010. Maximise your recoveries in a flash. Output Australia, vol. 27. pp. 4-7. [ Links ]
Curtin University Gold Technology Group. 2008. The interaction of flash flotation and gravity concentration. Back to Basics gold processing course, Curtin University, Perth. Unpublished work. [ Links ]
Drzymala, J. 1994. Hydrophobicity and collectorless flotation of inorganic materials. Advances In Colloid and Interface Science, vol. 50. pp. 143-185. [ Links ]
Dunne, R. 2005. Flotation of gold and gold-bearing ores. Developments In Mineral Processing: Advances In Gold Ore Processing. Wills, B.A. (ed.). Elsiever, Amsterdam, The Netherlands. pp. 309-343. [ Links ]
Kelebek, S. and Nanthakumar, B. 2007. Characterization of stockpile oxidation of pentlandite and pyrrhotite through kinetic analysis of their flotation. International Journal of Mineral Processing, vol. 84. pp. 69-80. [ Links ]
Kelly, E.G. and Spottiswood, D.J. 1989. Flotation and other surface separations. Introduction to Mineral Processing. John Wiley & Sons, Denver, Colorado. [ Links ]
Kelsall, D.F. 1961. Application of probability in the assessment of flotation systems. Transactions of the Institution of Mining and Metallurgy, vol. 70. pp. 191-204. [ Links ]
Laplante, A. and Dunne, R. 2002. The gravity recoverable gold test and flash flotation. Proceedings of the 34th Annual Meeting of the Canadian Mineral Processors, ottawa, Canada, 22-24 January 2002. [ Links ]
Laplante, A. and Staunton, W.P. 2005. Gravity recovery of gold - an overview of recent developments. Treatment of Gold Ores: Proceedings of the International Symposium on the Treatment of Gold Ores, Calgary, Alberta, Canada, 21-24 August 2005. Canadian Institute of Mining, Metallurgy and Petroleum. [ Links ]
Mackinnon, S., Yan, D., and Dunne, R. 2003. The interaction of flash flotation with closed circuit grinding. Minerals Engineering, vol. 16. pp. 1149-1160. [ Links ]
Malhotra, D. and Harris, L. 1999. Review of plant practices of flotation of gold and silver ores. Advances In Flotation Technologies. Parekh, B.K. and Miller, J.D. (eds.). Scociety for Mining, Metallurgy and Exploration, Littleton, Colorado. pp. 167-181. [ Links ]
McGrath, T.D.H., Staunton, W.P., and Eksteen, J.J. 2013. Development of a laboratory test to characterise the behaviour of free gold for use in a combined flash flotation and gravity concentrator model. Minerals Engineering, vol. 53. pp. 276-285. [ Links ]
Newcombe, B., Bradshaw, D., and Wightman, E. 2012. Flash flotation... and the plight of the coarse particle. Minerals Engineering, vol. 34. pp. 1-10. [ Links ]
O'Connor, C.T. and Dunne, R.C. 1994. The flotation of gold bearing ores - a review. Minerals Engineering, vol. 7. pp. 839-849. [ Links ]
Pevzner, M.L., Shcherbakov, V.I., and Kosova, L.Y. 1966. Behaviour of gold during grinding. Tsvetnaya Metallurgia, vol. 39, no. 5, pp. 11-12. (In Russian). [ Links ]
Sutherland, D.N. 1989. Batch flotation behaviour of composite particles. Minerals Engineering, vol. 2, no. 3. pp. 351-367. [ Links ]
Taggart, A.F. 1945. Handbook of Mineral Dressing. Wiley, New York. [ Links ]
Teague, A.J., Van Deventer, J.S.J., and Swaminathan, C. 1999. A conceptual model for gold flotation. Minerals Engineering, vol. 12, no. 9. pp. 1001-1019. [ Links ]
Tennyson, S. 1980. The hydrophilic nature of a clean surface. Journal of Colloid and Interface Science, vol. 75. pp. 51-55. [ Links ]
Trahar, W.J. 1981. A rational interpretation of the role of particle size in flotation. International Journal of Mineral Processing, vol. 8, no. 4. pp. 289-327. [ Links ]
Vieira, A.M. and Peres, A.E.C. 2007. The effect of amine type, pH, and size range in the flotation of quartz. Minerals Engineering, vol. 20, no. 10. pp. 1008-1013. [ Links ]
Wardell-Johnson, G., Bax, A., Staunton, W.P., Mcgrath, J., and Eksteen, J.J. 2013. A decade of gravity gold recovery. World Gold2013 Brisbane, Australia. Australasian Institute of Mining and Metallurgy, Victoria, Australia. pp. 225-232. [ Links ]
Wills, B.A. and Napier-Munn, T. 2006. Wills' Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery. 7th edn. Elsevier/Butterworth Heinemann, Amsterdam. [ Links ]
Zheng, X., Manton, P., Burns, F., Crawford, A., and Griffin, P. 2010. Operating strategies to maximise gold recovery at Telfer. Minerals Engineering, vol. 23, no. 15. pp. 1159-1166. [ Links ]














