Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.115 n.2 Johannesburg Feb. 2015
GENERAL PAPERS
Effect of inorganic chloride on spontaneous combustion of coal
Y.-B. TangI, II; Z.-H. LiII; Y.I. YangII; D.-J. MaII; H.-J. JiII
ICollege of Mining Technology, Taiyuan University of Technology, Taiyuan
IISchool of Safety Engineering, China University of Mining and Technology, Xuzhou
SYNOPSIS
Chlorine-containing minerals are commonly present in coal. Associated minerals such as pyrite can undergo exothermic reactions. Consequently, it is of great significance to study the effect of inorganic chloride on the spontaneous combustion of coal. In this study, the effects of five inorganic chlorides (sodium chloride, magnesium chloride, potassium chloride, calcium chloride, and zinc chloride) on the spontaneous oxidation of coal were investigated. Analysis of the gaseous products of coal oxidization at low temperatures (323K to 453K) showed that the presence of inorganic chlorine in coal markedly decreases O2 consumption and the generation of CO and CO2. Samples of raw coal and chlorine-loaded coal were oxidized for 36 hours under the same experimental conditions. Infrared diffuse reflectance spectroscopy results showed that inorganic chloride can inhibit the oxidative decomposition of some functional structure components (methyl, methylene, methine, and hydroxy) in the coal. The influence of inorganic chloride on the oxidation characteristics of the functional groups in coal during spontaneous combustion was investigated using benzyl alcohol and 1-phenyl propanol as model compounds, which were tested under the same experimental conditions as the coal samples. The oxygen consumption of model compounds with and without the addition of inorganic chloride further suggested that inorganic chloride may hinder the oxygenolysis of these structures during low-temperature oxidation. This phenomenon can be attributed to the radical reaction from the perspective of radical chemistry. It can therefore be concluded that inorganic chlorides play an inhibitory role in the spontaneous combustion of coal.
Keywords: coal, spontaneous combustion, inorganic chloride, gaseous products, model compounds, FTIR.
Introduction
Spontaneous combustion of coal is a serious problem that often occurs in the coal industry (Jones and Townend, 1945; 1949. Although several theories have been proposed to account for the phenomenon (Wang, 2006; Li, 1996; Wang, 1999; Lopez, 1998), the definitive mechanism of coal spontaneous combustion is still unknown. However, it is acknowledged that coal spontaneous combustion is a kind of oxidizing reaction (Pis et al., 2996; Itay, Hill, and Glasser, 1989). Hence, the relevant parameters during coal oxidation at low temperatures can be used to indicate the tendency of coal to spontaneously combust (Jones et al., 1998; Wang, Dlugogorski, and Kennedy, 2003).
Coal is a complex material consisting of combustible maceral and mineral components, and which contains dozens of minor elements besides various major elements including C, H, O, N etc. (Zhu, 2008). Furthermore, coal contains major impurity elements such as carbonates, sulphates, chlorides, and silicates, most of which are associated with the mineral components (Tang and Zhao, 2008; Zhang, 2009), and which are likely to influence the propensity of coal to undergo spontaneous combustion. For example, coal rich in pyrite is more likely to undergo spontaneous combustion, due to the presence of Fe2+ ions (Cole et al., 1987). Although the mechanism of this reaction has been studied extensively, there has been little work on the effect of other elements on coal spontaneous combustion to date. Many scholars have investigated the effect of adding different inorganic compounds to coal as possible fire retardants in order to control spontaneous combustion (Beamish and Arisoy, 2008; Carras and Young, 1994). A number of investigations have been carried out on the effect of mineral matter on coal liquefaction, coal char combustion, and coal pyrolysis etc. (Ma, 2011; Hanzade, Reha, and Aysegül, 1999; Li, Lu, and Jiao, 2009). However, there are few systematic studies of the influence of specific elements on coal spontaneous combustion.
Chlorine is a common trace element in coal, occurring mainly as an accompanying mineral (rock salt, potassium salt, bischofite, and hydrophilite etc.) Caswell, Holmes, and Spears, 1984; Vassilev, Eskenazy, and Vassileve, 2000). We therefore investigated the effects of five inorganic chlorides on coal spontaneous combustion. In addition, we tested model compounds under the same experimental condition as supporting research, in the light of coal molecular structure and organic chemistry (Benjamin, 1984; Shinn, 1984). Investigations using model compounds can provide references for showing the oxidation characteristics of the functional groups in coal during the process of spontaneous combustion (Li, Wang, and Song, 2009). Based on the macromolecular structural model of coal (Matthews, van Duin, and Chaffee, 2011), two kinds of model compounds were employed to investigate the effect of inorganic chloride on some specific active groups during the spontaneous combustion of coal.
Experimental
The test samples were collected from the no. 3 coal seam at Xutuan, Huaibei City, Anhui Province, China. The samples were crushed to a grain size of between 0.180 mm and approximately 0.250 mm for testing. The analysis (including macerals and minerals) of the no. 3 coal seam at Xutuan is shown in Table I. The additives, chemically pure (>99%) sodium chloride, potassium chloride, magnesium chloride, calcium chloride, and zinc chloride, were purchased from Sinopharm Chemical Reagent Co. Ltd. The samples were prepared by dissolving 0.05 mol of each reagent in 7 ml of deionized water and adding the solution to 100 g of coal sample with constant stirring.
The samples were allowed to stand in sealed containers for 24 hours in order for fully equilibrate the solution with the coal. Before each experiment, the coal sample was dried at 40°C for 12 hours in a vacuum drying oven (Shanghai Saiou Testing Equipment Co. Ltd) in order to exclude the interference of moisture with spontaneous combustion. 'Control' samples were prepared using the same procedure, but without the chloride addition. The samples used in the experiments can be divided into two groups: coal with inorganic chloride added and coal treated with deionized water only.
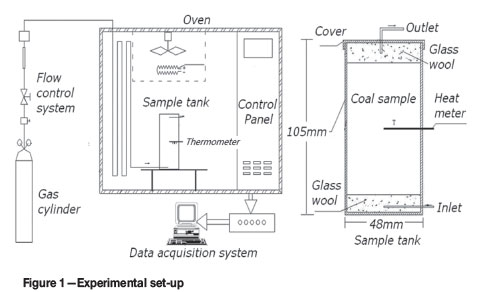
The experimental set-up is shown in Figure 1. The coal sample (40 g) was placed into the sample tank, which included two vent lines (inlet and outlet). The sample tank consisted of a cylindrical container with the height of 105 mm and diameter of 48 mm. The hole located in the centre of the sample tank was fitted with a temperature sensor, the top of which was in the geometric centre of the sample tank. Dry air at a flow rate of 20 ml/min was provided from a compressed gas cylinder. During the reaction, the sample tank was heated from 323K to 453K at a rate of 1K/min. The gaseous products were analysed by gas chromatography (SP501N-type, Beijing East & West Analytical Instruments Co. Ltd.).
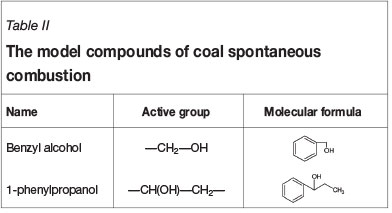
The influence of inorganic chloride on the oxidation characteristics of the functional groups in coal during spontaneous combustion was investigated using model compounds. Each selected model compound must contain only one representative oxidative active group, which should be a common structure of coal. According to the molecular structure, coal contains aromatic structures and functional groups such as hydroxyls and alkanes. Therefore, benzyl alcohol and 1-phenylpropanol were adopted as the model compounds in this experiment (Table II). Firstly, 0.02 mol of model compound was mixed with 10 g acetone, 0.01 mol inorganic chloride, and 40 g inert support (Figure 2). The parameters of the inert support are shown in Table III. This mixture was then dried for 12 hours in the vacuum drying oven to ensure that the acetone completely evaporated and that the model compound was uniformly attached to the inert support. After the abovementioned pretreatment, the model compounds were tested under the same experimental conditions as the coal samples.


In order to further elucidate the reaction principles governing the effects of inorganic chloride on spontaneous combustion of coal, the structural changes in the coal molecule before and after low-temperature oxidation were investigated using Fourier transform infrared spectroscopy (FTIR).
The coal samples were oxidized for 36 h under the above-mentioned experimental settings, then examined by infrared spectroscopy at a frequency in the range 400-4000 cm-1. Each sample was scanned 32 times.
Results and discussion
Analysis of gaseous products
As shown in Figure 3, the proportions of the gases produced by low-temperature oxidation of coal were varied by additions of inorganic chloride. During the entire process of low-temperature oxidation, the oxygen concentration in air flowing through the control sample, treated with only deionized water, decreased from 20.46% to 5.79% between 323K and 453K. For the samples loaded with inorganic chloride, the decrease was slower. Furthermore, the trends for sodium chloride and potassium chloride were similar, with the oxygen concentration falling to 15.03% and 16.08% respectively at a temperature of 453K. In the case of calcium chloride and zinc chloride, the oxygen concentration declined slowly until 373K, and then dropped sharply to 10.67% and 10.45%, respectively. Notably, the oxygen concentration with magnesium chloride decreased only slowly up to 373K, and then remained relatively stable at around 19.0% to 18.4%.
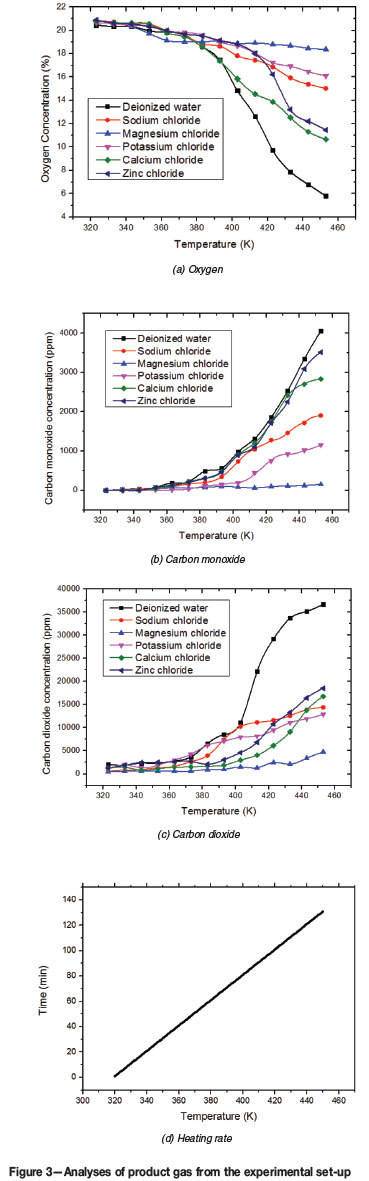
The CO concentration in the product gas from the low-temperature oxidation of coal increased from zero to 4056 ppm between 323K and 453K. The increase was less from coal treated with inorganic chlorides. For sodium chloride and potassium chloride additions, CO concentrations remained at a low level (below 200 ppm) until 383K and then rose rapidly to 1903 ppm and 1151 ppm respectively at a temperature of 453K. The upward trends for calcium chloride and zinc chloride were quite similar to that for coal treated with only deionized water, although the CO concentrations were slightly lower, diverging only when the reaction temperature reached 443K. The CO concentration for magnesium chloride remained low at 153 ppm throughout the entire reaction.
The CO2 concentrations generally follow the same trends as those for CO. The CO2 content of the product gas for magnesium chloride-treated coal was also lowest among the five chloride-treated samples. The CO concentrations from sodium chloride and potassium chloride are consistently higher those from calcium chloride and zinc chloride samples across the entire temperature range. However, at temperatures higher than 413K the CO2 concentrations from the calcium chloride and zinc chloride samples exceed those from sodium chloride and potassium chloride samples.
In summary, treatment with inorganic chloride decreased the oxygen consumption by the oxidation reaction at low temperatures, especially from 393K to 453K, and lower amounts of CO2 and CO were generated at the same reaction temperatures. The results suggest that inorganic chloride can effectively inhibit the low-temperature oxidation of coal.
The study shows that of the five chlorides tested, potassium chloride has a medium inhibitory effect on the low-temperature oxidation of coal. The relevant data for potassium chloride was therefore investigated using infrared spectroscopy and model compounds.
Infrared spectroscopy
The chief characteristic of the infrared spectrum is that the frequencies of vibration of the same types of chemical bonds are very similar and always appear within a certain range. Table IV and Figure 4 depict the attribution of the major peaks in the sample from seam no. 3 of Xutuan colliery, according to the coal chemistry and infrared spectroscopy (Speight, 1971).
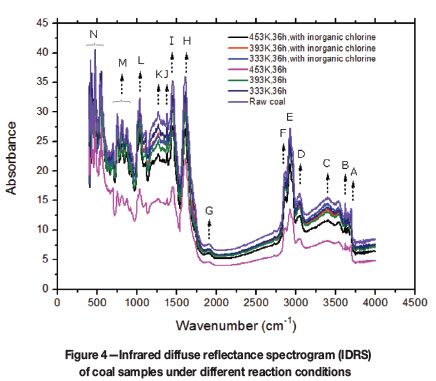
Figure 4 shows that oxidation for 36 hours results in a significant change in the organic structure of XT coal, which affects the tendency to undergo spontaneous combustion. This change is most apparent at temperatures between 333K and 453K.t At first, there is no apparent change of all the absorption peaks in the infrared spectra induced by the heating and oxidization at 333K. However, it is clear that after low-temperature oxidation at 453K, the peaks corresponding to -OH,-CH2-, and -CH3 are weakened, which suggests that the activity of these structures (methyl, methylene, methine, and hydroxy) in coal molecule is damaged to some extent. The peak value of the corresponding region of C-H (aromatic ring) and C=C (aromatic ring) decreases slightly, which indicates that the main structure of the aromatic ring or fused ring has not been destroyed during the low-temperature oxidation process. Under these conditions, apart from the absorption peak of the vibration of aromatic ring C=C and C-H bonds, all other absorption peaks decrease to varying degrees.
In comparison, after the addition of inorganic chloride, the functional groups on the surface of coal molecules change slightly. In general, there is no great change in the intensity of most of the absorption peaks, including the stretching vibrations of aromatic ring C=C and carbonyl O-H as the temperature passes 333K; while the absorption peaks of -CH2- and C-O decrease slightly after the XT coal is heated at 453K for 36 hours. This indicates that the inorganic chloride inhibits the oxidation and decomposition of some functional groups in the coal during low-temperature oxidation.
Model compounds
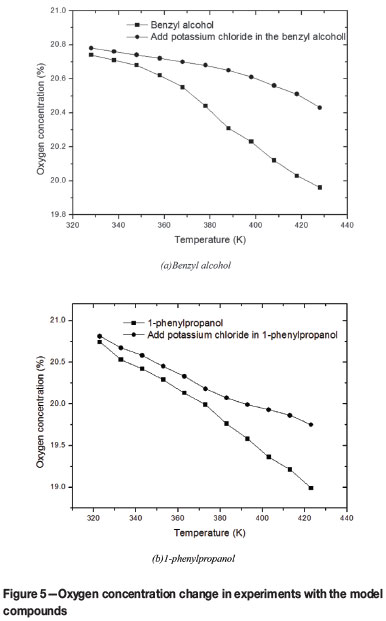
Similar to the coal molecular structrure (Shi, Deng, and Wang, 2004), the aromatic ring in the model compounds is relatively stable and the side chain is easily oxidized. It can be seen from Figure 5 that potassium chloride can inhibit the oxidation of benzyl alcohol and 1-phenylpropanol. During the oxidation of benzyl alcohol, the oxygen concentration in the product gas decreased from 20.74% to 20.55% between 328K and 368K. In particular, the concentration fell sharply to 19.96% when the temperature rose to 428K. However, after the addition of potassium chloride, the oxygen concentration declined more slowly, dropping to only 20.43% from 328K to 428K. Similarly, potassium chloride suppressed the oxidation of 1-phenylpropanol. Between 328K and 428K, the oxygen concentration in the product gas fell from 20.81% to 19.75% after loading potassium chloride into 1-phenylpropanol, compared with 20.74% to 18.99% without potassium chloride. These results suggest that inorganic chloride can inhibit the oxidation of methyl, methylene, methine, and hydroxy groups in model compounds to varying degrees.
Radical reactions
Spontaneous combustion of coal produces CO, CO2, and other products, which has been verified in underground and laboratory test work. This phenomenon can be explained by the chain-transfer of radicals. According to free radical theory, the initial stage of coal spontaneous combustion can be attributed to the radical reactions (Li, 1996). The low-temperature oxidation of coal can generate numerous free radicals, such as H*, OH*, and O*. The continuous cyclic generation of free radicals not only leads to spontaneous chain reactions, but also brings about heat accumulation in the coal body, which will eventually result in spontaneous combustion. However, with chlorine present in the coal, Cl* would be produced with increasing temperature. The reaction of Cl* and OH*, and of H* and O*, would inhibit spontaneous combustion.
The reactions are as follows:
HCl* + OH* → H2O + Cl*
HCl* + O* → OH* + Cl*
HCl* + H* → H2 + Cl*
The newly generated Cl* can further react with combustible materials. The continuous reaction is capable of removing considerable amounts of OH*, H*, and O*. As a result, chlorides can inhibit the spontaneous combustion of coal.
Conclusion
This study revealed that inorganic chloride can inhibit the spontaneous combustion of coal. The important indicators of the spontaneous combustion process, such as O2 consumption and production of CO and CO2, decreased significantly when inorganic chlorides were added to coal samples. Of the five reagents used in the investigation, magnesium chloride has the best inhibiting effect on the low-temperature oxidation of coal. We conclude that inorganic chloride can inhibit the oxidation of methyl, methylene, methine, and hydroxy groups in the low-temperature oxidation process of model compounds of coal. This phenomenon can be explained to some extent by the process of radical reaction. This may play an important role in coal spontaneous combustion if the raw coal has a high chlorine content. There is plenty of scope for extending this work to investigate the effect of other elements on coal spontaneous combustion.
Acknowledgement
This work was supported by the Project of China National Natural Science Foundation (No. 51074158) and the Fundamental Research Funds for the Central Universities (No. 2012LWBZ10).
References
Beamish, B.B. and Arisoy, A. 2008. Effect of mineral matter on coal self-heating rate. Fuel, vol. 87. pp. 125-130 [ Links ]
Benjamin, B.M. 1984. New chemical structural features of coal. Fuel, vol. 63. pp. 1340-1348. [ Links ]
Carras, J.N. and Young, B.C. 1994. Self-heating of coal and related materials: models, application and test methods. Progress in Energy and Combustion Sciences, vol. 20. pp. 1-15. [ Links ]
Caswell, S.A., Holmes, L.F., and Spears, D.A. 1984. Water-soluble chlorine and associated major cations from the coal and mudrocks of the Cannock and North Staffordshire coalfields. Fuel, vol. 63. pp. 774-781. [ Links ]
Cole, D.A., Simmons, G.W., Herman, R.G., Klier, K., and Czakó-Nagy, I. 1987. Transformations of iron minerals during coal oxidation. Fuel, vol. 66. pp. 1240-1248. [ Links ]
Hanzade, H., Reha, Y., and Aysegül, E. 1999. Effect of mineral matter on the reactivity of lignite. Thermochimica Acta, vol. 342. pp. 79-84. [ Links ]
Jones, J.C., Henderson, K.P., Littlefair, J., and Rennie, S. 1998. Kinetic parameters of oxidation of coals by heat-release measurement and their relevance of self-heating tests. Fuel, vol. 77. pp. 19-22. [ Links ]
Jones, R.E. and Townend, D. 1945. Mechanism of the oxidation of coal. Nature, vol. 155. pp. 424-425. [ Links ]
Jones, R.E. and Townend, D. 1949. The oxidation of coal. Journal of the Society of Chemical Industry, vol. 68. pp. 197-201. [ Links ]
Li, M., Lu, S., and Jlao, X.W. 2009. Experimental study on influence the included minerals on the char combustion characteristics. Coal Conversion, vol. 32. pp. 33-36. [ Links ]
Li, Z.H., Wang, Y.L., and Song, N. 2009. Experiment study of model compound oxidation on spontaneous combustion of coal. Procedia Earth and Planetary Science, vol. 1. pp. 123-129. [ Links ]
Li, Z.H. 1996. Mechanism of free radical reactions in spontaneous combustion of coal. Journal of China University of Mining and Technology, vol. 125. pp. 111-114. [ Links ]
Lopez, D. 1998. Effect of low-temperature oxidation of coal on hydrogen-transfer capability. Fuel, vol. 77. pp. 1623-1628. [ Links ]
Ma, Y.J. 2011. Thermodynamics simulation and experimental study of volatile trace elements in coal during combustion. Henan Polytechnic University, Jiaozuo. pp. 32-51. [ Links ]
Mathews, J.P., van Duin, A.C.T., and Chaffee, A.L. 2011. The utility of coal molecular models. Fuel Processing Technology, vol. 92. pp. 718-728. [ Links ]
Itay, M., Hill, C.R., and Glasser, D. 1989. A study of the low temperature oxidation of coal. Fuel Processing Technology, vol. 21. pp. 81-97. [ Links ]
Pis, J.J., del la Puente, G., Fuente, E., MorAn, A., and Rubiera, F. 1996. A study of the self-heating of fresh and oxidized coals by differential thermal analysis. Thermochimica Acta, vol. 279. pp. 93-101. [ Links ]
Shi, T., Deng, J., and Wang, X.F. 2004. Mechanism of spontaneous combustion of coal at initial stage. Journal of Fuel Chemistry and Technology, vol. 32. pp. 6-8. [ Links ]
Shinn, J H. 1984. Study of coal molecular structure. Fuel, vol. 63. pp. 83. [ Links ]
Speight, J.G. 1971. The application of spectroscopic technique to the structural analysis of coal and petroleum. Applied Spectroscopy Reviews, vol. 15. pp. 211-264. [ Links ]
Tang, X.Y. and Zhao, J.Y. 2002. Modes of occurrence of trace elements in coal. Coal Geology of China, vol. 14. pp. 14-17. [ Links ]
Vassilev, S.V., Eskenazy, G.M., and Vassileva, C.G. 2000. Contents modes of occurrence and origin of chlorine and bromine in coal. Fuel, vol .79. pp. 903-921. [ Links ]
Wang, D.M. 2006. Mine Fire. China University of Mining and Technology Press, Xuzhou. pp. 47-51. [ Links ]
Wang, H.H., Dlugogorski, B.Z., and Kennedy, E.M. 2003. Coal oxidation at low temperatures: oxygen consumption, oxidation products, reaction mechanism and kinetic modelling. Progress in Energy and Combustion Science, vol. 29. pp. 487-513. [ Links ]
Wang, H.H. 1999. Theoretical analysis of reaction regimes in low-temperature oxidation of coal, Fuel, vol. 78. pp. 1073-1081. [ Links ]
Zhang, H. 2009. Study on the influence of mineral matters on the combustion characteristics and kinetics of pulverized coals. Journal of China University of Mining and Technology, vol. 8. pp. 451-456. [ Links ]
Zhu, Y.H. 2008. Coal Chemistry. Chemical Industry Press, Beijing. pp. 129-137. [ Links ]














