Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.114 n.11 Johannesburg Nov. 2014
GENERAL PAPERS
Liquid-liquid extraction and separation of copper(II) and nickel(II) using LIX®984N
N.B. Devi; B. Nayak
Department of Chemistry, Institute of Technical Education and Research, Siksha 'O'Anusandhan University, India
SYNOPSIS
The extraction of copper(II) from sulphate solution was investigated using LIX®984N in kerosene. The parameters that could affect the extraction, such as equilibrium pH, extractant concentration, salt concentration, various diluents, and temperature, were separately investigated. On the basis of slope analysis, the complex formed in the organic phase is proposed to be CuR2, where R is the anionic part of LIX®984N. The extraction equilibrium constant and Gibbs free energy (AG) of the extraction process were determined. The positive value of AH obtained from temperature variation studies showed that the extraction process is endothermic. The extraction of copper was lowest when chloroform was used as the diluent. Separation and recovery of copper from a synthetic copper-nickel solution containing 6.35 g/L Cu(II) and 0.58 g/L Ni(II) was carried out using 20%(v/v) LIX® 984N. The separation factor (β = ZWDmí) was found to be pH-dependent. A two-stage batch countercurrent extraction was carried out at an O:A ratio of 3:4 at equilibrium pH 1.05 for extraction of copper from copper-nickel solution, followed by copper stripping from the loaded organic phase with 15% H2SO4 at an O:A ratio of 3:1.
Keywords: copper, nickel, LIX® 984N, solvent extraction, stripping.
Introduction
In recent years, the demand for copper and nickel has risen world-wide due to their use in printed circuit boards, batteries, application in marine alloys (due to excellent resistance to corrosion by seawater), and a wide range applications in day-to-day life (Doebrich, 2009). Increasing demand for these metals is leading to rapid depletion of high-grade resources. Alternative sources, such as e-wastes, lithium ion batteries, copper converter slags, polymetallic nodules etc., are needed to meet future demand. The copper-nickel system was chosen for this investigation because some slags (copper converter slag, anode slag of Hindustan Copper, and by-products of UCIL Jaduguda) contain high amounts of both copper and nickel. Hydrometallurgical routes play a vital role in the processing of these secondary sources. Solvent extraction is a well-established technique for the removal and separation of various metal ions. About 30% of the world's copper is produced by acid leaching, solvent extraction (SX), andelectrowinning (Cox, 2004). Hydroxyoximes are well-known extractants which are widely used for copper extraction from dilute acidic sulphate solutions (Ritcey and Ashbrook, 1979; Szymanowski, 1993; Szymanowski, 1990).
Only two classes of extractants, i.e. ketoximes and aldoximes, have gained commercial acceptance as reagents for solvent extraction from acidic leach solutions. Oxime mixtures have been shown to be advantageous over the individual group ketoximes and aldoximes (Kordosky et al., 1985). Rodriguez et al. (1997) studied the extraction of several metal ions using LIX® 984, which is a 1:1 volume blend of LIX® 860 and LIX® 84 in n-heptane. They reported the equilibrium constant values for the extraction reactions, and proposed the species extracted into the organic phase. Fouad (2007) studied the extraction equilibria of copper(II) with Cyanex 301, LIX® 984N, and mixtures of these two reagents. The enthalpy change (AH) of the individual extractants and their mixtures was determined and the endothermic process elucicated. Kul and Cetinkaya (2009) developed a complete hydrometallurgical process on a laboratory scale for recovering copper from copper electroplating second rinse bath liquor containing 2.5 g/L copper using LIX® 984N-C in kerosene. Recovery of copper from waste printed circuit boards (PCBs) by nitric acid leaching and extraction using LIX® 984N was reported by Long Le et al. (2011). They first studied the co-extraction of other metals (Pb, Zn, Fe, and Ni) from a diluted leach liquor with 10% LIX® 984N, and found that the co-extraction of these metals was negligible up to pH 1.9, except for iron (6.4%). Based on these results, they used 50% LIX® 984N to recover all the copper from the actual leach liquor of composition 42.11 g/L Cu, 2.12 g/L Fe, 4.02 g/L Pb, 1.58 g/L Zn, and 0.48 g/L Ni at an A:O ratio of 1:1.5 in three stages and at pH 1.5. Sulphuric acid (H2SO4 -360 g/L) was used for stripping. Qing-ming et al. (2008) studied the separation of copper and iron from dump bioleaching solution from Dexing Copper Mine using LIX® 984N. Kinoshita et al. (2003) reported the separation of copper and nickel from nitrate media containing 11 180 mg/L Cu and 1160 mg/L Ni using 200 g/L LIX® 984. Extraction and separation of copper and nickel by LIX® 984N from ammoniacal medium was investigated by Sridhar et al. (2009). They found that both the metals co-extracted over the pH range 7 to 9. A selective stripping method was used to separate copper and nickel.
The use of LIX® 984N has received attention for extracting copper from copper-rich leach liquors. There are few reports available regarding equilibrium studies of the copper-LIX® 984N system (Aminian and Bazin, 2000) and the separation of copper and nickel from sulphate medium (Ochromowicz and Chmielewski, 2013). The present study investigates the extraction equilibrium in the copper-LIX® 984N system, the species extracted into the organic phase, and evaluates the thermodynamic parameters in addition to diluent and salt effects. McCabe-Thiele plots and batch countercurrent extractions were also been carried out to study the separation and recovery of copper from a synthetic copper and nickel solution.
Experimental
Solutions and reagents
Stock solutions of copper(II) and nickel(II) (1M each) were prepared by dissolving the sulphate salts in double-distilled water. LIX® 984N (a mixture of 5-nonylsalicylaldoxime and 2-hydroxy-5-nonylacetophenone oxime) was supplied by Cognis Inc. and was used without further purification. Distilled kerosene was used as diluent for the organic phase. All other reagents used were of analytical reagent grade.
Experimental methods
Solvent extraction
The metal-bearing solution was equilibrated with an equal volume of LIX® 984N for 5 minutes in a separating funnel. The pH of the aqueous solution before extraction was adjusted by adding dilute H2SO4 or NaOH solution. After phase separation the aqueous phase was collected and the equilibrium pH was measured. The metal content in the aqueous phase was determined by the thiosulphate method using starch as the indicator (Bassett et al., 1984). When both copper and nickel were present, the aqueous phase was analysed by atomic absorption spectrophotometry (AAS) using an ELICO-type instrument. The concentration of the metal ion in the organic phase was calculated by the difference in concentration before and after extraction. When required, the organic phase metal concentrations were determined after filtration through 1PS phase separating paper and stripping with 20% H2SO4, followed by analysis with AAS. All the extraction and stripping experiments were carried out at 30±1°C, except for the temperature variation study.
Extraction and stripping isotherms
While keeping the total volume of the aqueous and the organic phases constant, the solutions at different O:A ratios were shaken for 5 minutes at 30±1°C. After the extraction process, the metal concentrations in the raffinate (R) were determined by AAS and the metal concentration in the loaded organic (LO) phase was calculated by mass balance. After the stripping process, the metal concentration in the spent organic (SO) was determined by AAS and the metal in the aqueous calculated by mass balance. McCabe-Thiele constructions were drawn for the extraction and stripping isotherms.
Countercurrent SX process
A two-step countercurrent extraction process was simulated by batch experiments using a synthetic solution containing copper (6.35 g/L) and nickel (0.58 g/L) up to five cycles at an O:A ratio of 3:4. In each step, 80 mL of aqueous phase and 60 mL of organic phase (20% (v/v) LIX® 984N) were mixed in a separating funnel for 5 minutes. A schematic representation of countercurrent steps is given in Figure 1. The raffinates and the loaded organic phases (after stripping with H2SO4) were analysed by AAS. Similar steps were carried out for the batch countercurrent stripping process, which involves three stages at an O:A ratio of 3:1.
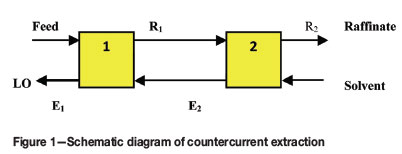
Results and discussion
Extraction of copper using LIX 984N
Effect of equilibrium pH
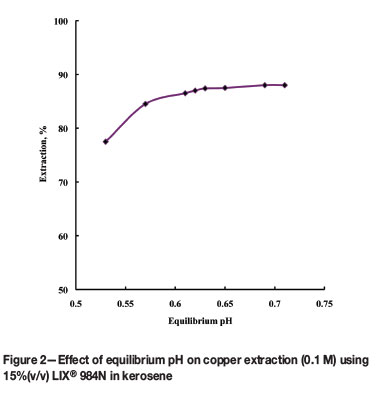
Experiments were carried out at room temperature to study the effect of equilibrium pH on the extraction of 0.1M Cu(II) using 15% (v/v) LIX® 984N in kerosene. The results are presented in Figure 2 as percentage of copper extraction versus equilibrium pH. As expected, the percentage extraction increased with increasing equilibrium pH; however, it remained practically constant at equilibrium after sometime. At an equilibrium pH of 0.53, the percentage copper extraction into the organic phase was 78%, whereas 87% copper was extracted at equilibrium pH 0.61 and 88% at equilibrium pH 0.71. This behaviour was also reported by Asghari et al. (2009) and Rodriguez et al. (1997), who found that copper extraction increased with increasing equilibrium pH to a certain value, after which it was independent of pH variation. In this work, the maximum extraction of copper was 88% (equilibrium pH range 0.63 to 0.71) with 15% LIX®984N. These extraction and equilibrium pH values are low compared to the values reported by Asghari et al. (2009). One reason may be the higher initial copper concentration - 6.35 g/L used in this study, compared with 2.5 g/L used by Asghari et al. Due to the availability of more copper ions, extraction is favoured and the release of more H+ ions results in a decreasing equilibrium pH value.
Effect of extractant concentration
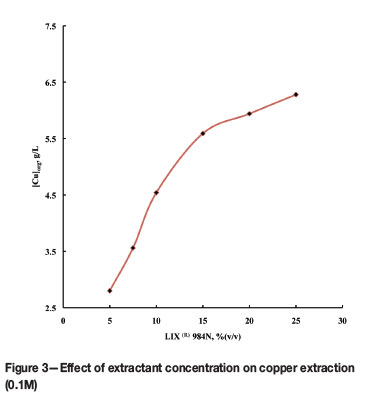
The effect of extractant concentration on copper extraction was studied by contacting sulphate solutions containing 0.1M copper(II) at an initial pH value of 3.95 with LIX® 984N at concentrations from 5-25% (v/v) . As shown in Figure 3, copper extraction increases from 2.8 g/L (44%) to 6.27 g/L (98.7%) with increase in extractant concentration from 5% to 25% (v/v) LIX® 984N.
Extraction mechanism
As LIX® 984N is a chelating extractant, it will form neutral complexes with copper(II) coordinating with the nitrogen atom of the oxime group and releasing two H+ ions into aqueous solution. The extraction equation can be written as:
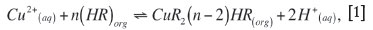
where n is the solvation number of the extractant, LIX® 984N.
The extraction equilibrium constant can be represented
as:
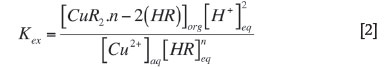
The distribution ratio (D) is the ratio of metal concentration in organic phase to the metal concentration in aqueous phase at reaction equilibrium. Substituting D in Equation [2] yields

Therefore,
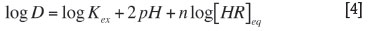
The concentration of copper chosen for the present study is 0.1M, and the results of the pH variation study led to the consideration of the equilibrium concentration of the organic phase (free extractant concentration remained after equilibration). This can be calculated as:
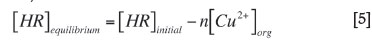
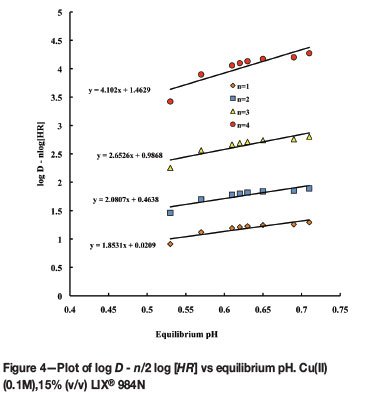
The use of various concentrations of LIX 984 to determine the solvation number was reported by Rodriguez et al. (1997), Aminian and Bazin (2000), and Fouad (2009). The plot of log D - nlog [HR]eq versus equilibrium pH for different values of the number of extractant molecules (n) is shown in Figure 4. The line that has a slope of approximately 2.0 has been selected. The slope is equal to the number of H+ ions in Equation [1].
Based on the above results, the extraction equilibrium reaction is written as:
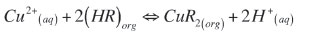
Buketova (2009) analysed the IR spectrum and confirmed that the structure of this CuR2 complex with LIX® 984N is non-polar. The intercept of the graph in Figure 4 is the logarithmic value of the extraction equilibrium constant. The equilibrium constant (Kex) for the above equilibrium is therefore 2.90 and the Gibbs free energy of the extraction is -2.69 kJ mol-1 (T = 303 K).
Effect of different media
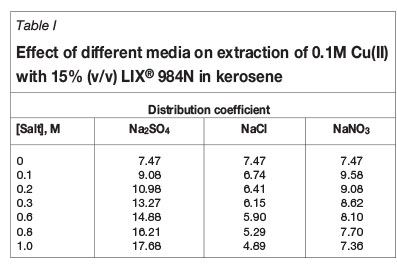
Some metals are recovered via a hydrometallurgical route consisting of three unit operations - leaching, SX, and electrowinning. The SX process depends mainly on the nature of the leach liquor. In addition to leach liquors in a single medium, recovery of metals from mixed-medium leach liquors are also of importance nowadays (Sarangi et al., 2007; El-Hefny et al., 2010). Sarangi et al. studied the separation and recovery of iron(III), copper(II), and zinc(II) from mixed sulphate and chloride media, whereas El-Hefny et al. used sulphate/thiocyanate media for zinc and cobalt separation. Keeping this in mind, the extraction of 0.1M Cu(II) was carried out with 15% (v/v) LIX® 984N in the presence of different salts such as sodium sulphate, sodium chloride, and sodium nitrate over the concentration range 0.1 to 1M. The results showed that the distribution coefficient of copper increased with increasing sodium sulphate concentration from 0.1M (D=9.08) to 1 M (D=17.68), but decreased with increase in sodium chloride concentration. In presences of 0.1M sodium nitrate, the distribution coefficient is more than the distribution coefficient value when sodium nitrate was absent, but it decreased constantly with further increase of NaNO3 concentration (Table I). However, the decrease is more significant with sodium chloride than with sodium nitrate. The decrease in extraction may be due to a salting-out effect.
Effect of diluent
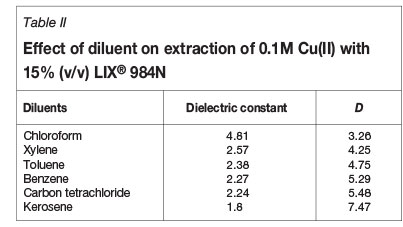
The choice of diluent is an important aspect of successful SX operation. A diluent reduces the viscosity of the extractants, but sometimes the nature of diluent affects the extraction process. To investigate this, the extraction of copper(II) with 15% (v/v) LIX® 984N was carried out using different diluents such as kerosene, benzene, xylene, toluene, carbon tetrachloride, and chloroform. The result showed that copper extraction depends on the dielectric constant value of the diluent - the higher the value of the dielectric constant, the lower the percentage extraction of copper (Table II). The reason for this is that with an increase in the dielectric constant of the diluent, the interaction between the diluent and the extractant is increased, thus decreasing the availability of the extractant for extraction of copper. The same trend was observed by Reazai and Nedjate (2003) and El-Nadi (2010) while studying the effect of diluents on the extraction of nickel and rare earths.
Effect of temperature
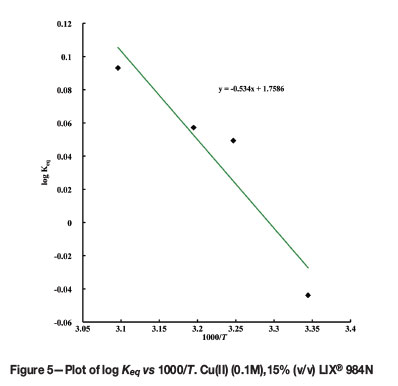
The extraction efficiency of certain extractants depends significantly on temperature, and therefore the extraction of 0.1M Cu(II) was studied at different temperatures (299-323K) with 15%(v/v) LIX® 984N in kerosene. It was observed that with increasing temperature the distribution ratio of copper extraction increased from 7.35 (299K) to 9.08 (323K). This indicates that the extraction is endothermic. The thermodynamic parameters such as enthalpy change (AH) and entropy change (AS) were calculated by plotting log Keq against 1000/T (Figure 5). From the plot, AH and AS were calculated and found to be 10.224 kJ mol-1 and 4.05 J K-1 mol-1 respectively. Aminian and Bazin (2000) also reported that the extraction of copper with LIX® 984 was endothermic, with a AH of 5.88 kJ mol-1 under the experimental conditions used (0.94 g/L Cu, pH = 2 and O:A = 0.1). Although in both cases the process was found to be endothermic, the difference in ΔH values may be due to the different experimental conditions.
Solvent extraction behaviour of copper in presence of nickel
To study the extraction behaviour of copper in presence of nickel, an aqueous solution containing 0.1M Cu(II) (6.35 g/L) and 0.01M Ni(II) (0.58 g/L) was chosen. Copper extraction was 98% with 25% (v/v) LIX® 984N, and 93% with 20% (v/v) LIX® 984N. In an industrial application the viscosity of the solvent should not be very high, therefore 20% (v/v) LIX® 984N in kerosene was selected to study copper-nickel extraction. Figure 6 represents the percentage extraction of Cu(II) and Ni(II) as a function of equilibrium pH of the aqueous solution. Extraction of copper increased from 61% to 91% with an increase in equilibrium pH from 0.36 to 0.91, while the extraction of nickel was within 6% to 8% over the equilibrium pH range 0.56 to 1.08. Kinoshita et al. (2003) reported the extraction of nickel to be 3-4 % around that range of equilibrium pH in the presence of copper. This result demonstrated that copper can easily be separated from nickel. The separation factor (β = DCu / )½) was calculated and is tabulated in Table III with respect to equilibrium pH. The separation factor was the highest (123.8) at equilibrium pH 0.95 and the lowest (63.3) at equilibrium pH 0.56.
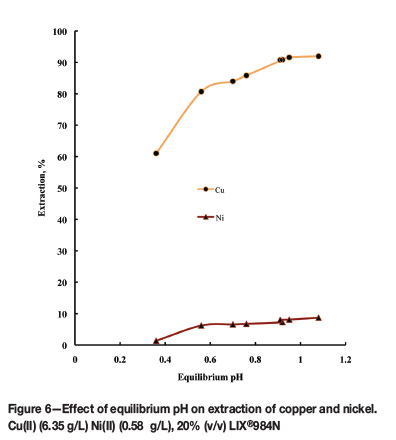
Extraction isotherms for copper and nickel and McCabe-Thiele plots
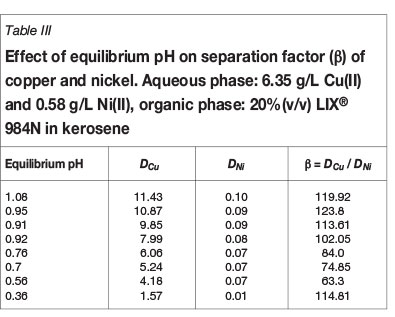
An aqueous solution containing 6.35 g/L copper(II) and 0.58 g/L nickel(II) with an initial pH of 3.95 was used for the extraction of copper with 20% (v/v) LIX®984N in kerosene. To determine the number of stages required for the extraction of copper, a McCabe-Thiele plot was constructed for O:A ratios from 1:5 to 5:1, with the total volume of the phases kept constant. The data in Figure 7 indicates that < 0.045 g/L copper will remain in the raffinate after two extraction stages at an O:A ratio of 3:4, which suggests that it could be possible to upgrade the copper concentration in the organic phase.
This prediction was confirmed by carrying out a two-stage batch countercurrent simulation study of up to five cycles at an O:A phase ratio of 3:4, in which the partially loaded organic from extraction stage 2 was fed to extraction stage 1. Analysis of copper and nickel values in the aqueous and organic phase confirmed the extraction of 8.4 g/L copper
with negligible nickel co-extraction (0.003 g/L) in the loaded organic phase (Table IV). Thus, a loaded organic phase containing 8.4 g/L copper was generated.
Stripping of copper
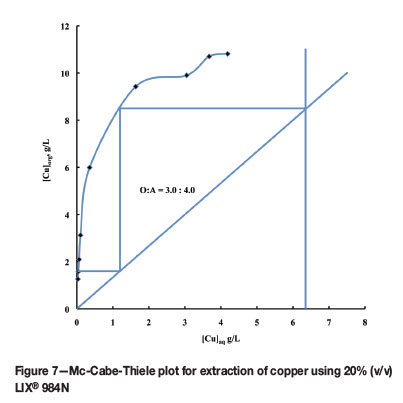
The copper-loaded organic phase was stripped with different concentrations of sulphuric acid (5-25%) at equal phase ratios for 5 minutes. The aqueous phase was diluted and analysed for copper. The percentage copper stripping increased from 67.4% with 5% H2SO4to 100% with 25% H2SO4. A McCabe-Thiele plot was constructed for the 20% loaded organic phase using 15% H2SO4 (98.2% stripping), with O:A phase ratios in the range 1:5 to 5:1. The data in Figure 8 suggests three stages of stripping at an O:A ratio of 3:1. A three-stage batch countercurrent experiment was carried out at the above O:A ratio using 15% H2SO4strip solution. The analysis of the aqueous phase showed an upgrading of copper to 25.1 g/L with 0.0089 g/L Ni in the loaded strip solution, leaving only 0.002 g/L copper in the organic phase (Table V).
Conclusions
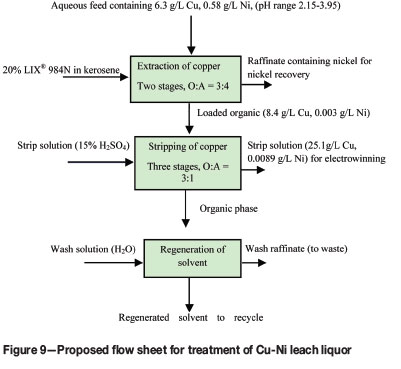
The extraction of 0.1M Cu(II) from sulphate solution using 15% (v/v) LIX® 984N showed a pH dependence up to 0.61, after which it is independent of equilibrium pH. The extraction of copper increased with increasing extractant concentration. The species extracted into the organic phase is proposed to be CuR2. The equilibrium constant and Gibbs free energy were found to be 2.9 and 2.69 kJ mol-1 respectively. The percentage extraction of copper increased with increasing sodium sulphate concentration. With increasing dielectric constant of different diluents, the copper extraction was found to decrease. Effective separation of copper and nickel from an aqueous feed solution containing 6.35 g/L copper(II) and 0.58 g/L nickel(II) could be achieved with 20% (v/v) LIX® 984N in kerosene in the initial pH range (2.15-3.96), corresponding to an equilibrium pH of 0.91-1.08). A two-stage batch countercurrent extraction experiment at an O:A ratio of 3:4 resulted in upgrading the copper from 6.35 g/L in the feed solution to 8.4 g/L in the loaded organic phase. Using 15% H2SO4 as the stripping agent, 25.1 g/L copper(II) was stripped into the loaded strip liquor, which could be suitable for electrowinning. A flow sheet is proposed for separation of copper and nickel (Figure 9) that could be used for treatment of sulphate leach liquors obtained from pressure leaching of copper converter slags, nickel smelter slags, and copper sulphide concentrates.
Acknowledgements
The authors thank Cognis, Ireland Mining Chemical Technology Ltd, (a division of BASF Chemicals) for providing the LIX® 984N. This research work was carried out with the encouragement and support of the authorities of Siksha'O'Anusandhan University.
References
Aminian, H. and Bazin, C. 2000. Solvent extraction equilibria in copper(II)-iron(III)- LIX® 984 system. Minerals Engineering, vol. 13, no. 6. pp. 667-672. [ Links ]
Asghari, H., Safarzadeh, M.S., Asghari, G., and Moradkham, D. 2009. The effect of impurities on the extraction of copper from sulfate medium using LIX®984N in kerosene. Russian Journal of Non-Ferrous Metals, vol. 50, no. 2. pp. 89-96. [ Links ]
Bassett, J., Denney, R.C., Jeffery, G.H., and Mendham, J. 1984. Vogel's Text Book of Quantitative Inorganic Analysis, 4th edn. Longman, UK. 379 pp. [ Links ]
BUKETOVA, A.E. 2009. An IR-spectroscopic examination of copper- LIX® 984N extractant complexes. Russian Journal of Applied Chemistry, vol. 82, no. 1. pp. 23-26. [ Links ]
COX, M. 2004. Solvent extraction in hydrometallurgy. Solvent Extraction Principles and Practice. Rydberg, J., Cox, M., Musikas, C., and Choppin, G.R. (eds.). Marcel Dekker, New York. Chapter 11, p. 454. [ Links ]
DOEBRICH, J. 2009. Copper - A Metal for Ages. US Geological Survey Fact Sheet 3031. http:/pubs.usgs.gov/fs/2009/3031 [ Links ]
El-Hefny, N.E., Gasser, M.S., Rizk, S.E., Saad, E.A., and Daoud, J.A. 2010. Separation and recovery of zinc(II) and cobalt(II) from a mixed sulfate/thiocyanate solution using some commercial organophosphorus extractants. Solvent Extraction and Ion Exchange, vol. 28. pp. 244-266. [ Links ]
El-Nadi, Y.A. 2010. Effect of diluents on the extraction of praseodymium and samarium by Cyanex 923 from acidic nitrate medium. Journal of Rare Earths, vol. 28, no. 2. p. 215. [ Links ]
Fouad, E.A. 2009. Separation of copper from aqueous sulfate solutions by mixtures of Cyanex 301 and LIX® 984N. Journal of Hazardous Materials, vol. 166, no. 2-3. pp. 720-727. [ Links ]
Kinoshita, T., Akita, S., Kobayashi, N., Kawaizumi, F., and Takahashi, K. 2003. Metal recovery from non-mounted printed wiring boards via hydrometallurgical processing. Hydrometallurgy, vol. 69, no. 1-3. pp. 73-79. [ Links ]
Kordosky, G.A., Olfason, S.M., Lewis, R.G., Defnner, V.L., and House, J.E. 1987. A state-of-the-art discussion on the solvent extraction reagents used for the recovery of copper from dilute sulfuric acid leach solutions. Separation Science and Technology, vol. 22, no. 2-3. pp. 215-232. [ Links ]
Kul, M. and Cetinkaya, U. 2009. Recovery of copper by LIX® 984N-C from electroplating rinse bath solution. Hydrometallurgy, vol. 98. pp. 86-91. [ Links ]
Long Le, H, Jeong, J., Lee, J-C., Pandey, B.D., Yoo, J-M., and Huyunh, T.H. 2011. Hydrometallurgical process for copper recovery from waste printed circuit boards (PCBs). Mineral Processing and Extractive Metallurgy Review, vol. 32. pp. 90-104. [ Links ]
Ochromowicz, K. and Chmielewski, T. 2013. Solvent extraction of copper(II) from concentrated leach liquors. Physicochemical Problems Of Mineral Processing, vol. 49, no. 1. pp. 357-367. [ Links ]
Qing-Ming, L., Run-Lan, Y., GuanU-Zhu, Q., Zheng, F., Ali-Liang, C., and Zhong-Wei, Z. 2008. Optimization of separation processing of copper and iron of dump bioleaching solution by LIX® 984N in Dexing Copper Mine. Transactions of the Nonferrous Metallurgical Society of China, vol. 18. pp. 1258-1261. [ Links ]
Reazai, K. and Nedjate, H. 2003. Diluent effect on the distribution ratio and separation factor of Ni(II) in the liquid-liquid extraction from aqueous acidic solutions using dibutyldithiphosphoric acid. Hydrometallurgy, vol. 68. pp. 11-21. [ Links ]
Ritcey, G.M. and Ashbrook, A.W. 1979. Solvent Extraction, Principles and Practices, Part II. Elsevier, Amsterdam. [ Links ]
Rodriguez De San Miguel, E., Aguilar, J.C., Bernal, J.P., Ballinas, M.L., Rodriguez, M.T.J., De Gyves, J., and Schimmel, K. 1997. Extraction of Cu(II), Fe(IIi), Ni(II), In(III), Co(II), Zn(II) and Pb(II) with LIX® 984 dissolved in n-heptane. Hydrometallurgy, vol. 47. pp. 19-30. [ Links ]
Sarangi, K., Parhi, P.K., Padhan, E., Palai, A.K., Nathsarma, K.C., and Park, K.H. 2007. Separation of iron(III), copper(II) and zinc(II) from a mixed sulphate/chloride solution using TBP, LIX® 84I and Cyanex 923. Separation and Purification Technology, vol. 55. pp. 44-49. [ Links ]
Sridhar, V., Verma, J.K., and Kukar, S.A., 2009. Selective separation of copper and nickel by solvent extraction using Lix® 984N. Hydrometallurgy, vol. 99., pp. 124-126. [ Links ]
Szymanowski, J. 1990. Copper Extraction with Hydroxyoximes. PWN, Warsaw. [ Links ]
Szymanowski, J. 1993. Hydroxyoximes and Copper Hydrometallurgy. CRC Press, Boca Raton. FL. p. 77. [ Links ] ♦
© The Southern African Institute of Mining and Metallurgy, 2014. ISSN2225-6253. Paper received Apr. 2013; revised paper received Sep. 2014.














