Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.114 n.11 Johannesburg Nov. 2014
MINING ENVIRONMENT AND SOCIETY CONFERENCE 2013
Translocation of an endangered succulent plant species from sandstone outcrops earmarked for coal mining
E. HarrisI; S.J. SiebertI; J.H.L. SmitII; J. van den BergI
IUnitfor Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
IIExxaro Resources, Service Department, Pretoria, South Africa
SYNOPSIS
Frithia humilis is an endangered succulent plant species. Its distribution range overlaps with the coal fields of Mpumalanga and it is therefore threatened by opencast mining activities in particular. One of the 11 known populations of F. humilis was translocated from a licensed coal mining site to three suitable receptor sites within the species' distribution range. A long-term monitoring programme was initiated to track the progress of the newly established populations. Temporal trends in population demography, size classes, and fecundity were recorded. Population numbers of size classes fluctuated annually. However, flower frequency increased over time and seedling recruitment contributed significantly to population growth. Receptor sites with similar geological conditions to the donor site had more persistent cohorts, which suggest that such sites should receive priority during the translocation of endangered edaphic specialists. This study not only confirms that a Frithia humilis population can successfully establish after a translocation, but also serves as an important baseline for future comparative purposes to gauge the long-term success of the translocation effort.
Keywords: edaphic specialist, endangered, Frithia, mining, relocation, succulent, translocation.
Introduction
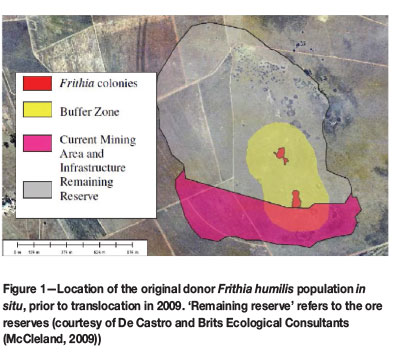
During 2008, an ecological survey by De Castro and Brits Ecological Consultants confirmed the presence of Frithia humilis Burgoyne, an endangered succulent plant species, at Inyanda Coal Mine (Exxaro) in Mpumalanga, where mining activities had already begun (McCleland, 2009). Mining activities provide the major threat to the persistence of this species, as coal deposits often underlie its rocky habitat (Cairncross, 2001); these sandstone outcrops are typically of the Dwyka and Ecca Groups, Karoo Supergroup (Burgoyne, Smith, and Du Plessis, 2000). Remaining natural populations of this plant species are at imminent risk from complete habitat transformation or loss.
The Inyanda Coal Mine's activities (Figure 1) threatened one of 11 naturally occurring populations of F. humilis (Burgoyne and Krynauw, 2005). As the mining license had already been granted before the species was found, translocation to three suitable receptor sites was carried out in 2009 as a mitigation attempt to save the population from extirpation (Figure 2). The translocation was performed by Inyanda Coal Mine, in collaboration with the South African National Biodiversity Institute (SANBI) and the Mpumalanga Tourism and Parks Agency (Burgoyne and Hoffman, 2011).

Translocation is the process whereby a population of living organisms is deliberately moved from one area to another suitable habitat within its existing distribution range (IUCN, 1987, 1998). Most often, such facilitated species movements aim to rescue individuals/populations from extinction, to restore populations, ecosystems, or habitats, o to conduct research on a species (Gordon, 1994).
Translocation can be successful only if a viable, self-sustaining population is established (Griffith et al., 1989). Such a population should be able to reproduce successfully to ensure long-term survival/persistence (Jusaitis, Polomka, and Sorensen, 2004). Certain 'milestones' can be indicative of translocation success: seedling recruitment, plant growth, and plant reproduction (Menges, 2008).
Although translocation is increasingly being viewed as a suitable conservation method for endangered species (Seddon, 2010), the financial and genetic risks undertaken are great (Milton et al., 1999). Translocations are infamous for their low probability of success (Mueck, 2000) and present a significant disturbance to the translocated population (Fahselt, 2007).
Gordon (1994) emphasised that translocation should never be considered as a substitute for the conservation of in situ populations and natural habitats. However, as a mitigation measure, as in the case of the imperilled F. humilis population, translocation is becoming an essential conservation intervention where no other options are available.
The main aim of the F. humilis translocation discussed in this paper was to investigate whether the re-establishment of a threatened population elsewhere could act as mitigation, especially as the threatened species is an edaphic specialist (Burgoyne, Krynauw, and Smith, 2000). Essentially, this study provides a contribution to the evaluation of the viability of translocation as a conservation strategy in the South African conservation context, given that translocation has only been selectively applied nationally (Milton et al., 1999). This study also contributes to scientific knowledge about species translocation.
Methods
Study species
Frithia humilis, commonly referred to as 'fairy elephant's feet', is a rare and endangered (Burgoyne and Krynauw,
2005) window plant (Figure 3A) in the succulent 'vygie' family, namely the Aizoaceae/Mesembryanthemaceae (Burgoyne, Krynauw, and Smith, 2000). This diminutive succulent, rarely protruding more than 30 mm above ground, has retracting leaves, which enables the entire plant to withdraw beneath the soil during the dry winter months (Moore, 1986) (Figure 3B). Consequently, the plant is protected from desiccation and other environmental factors (Burgoyne, Smith, and Du Plessis, 2000). It is therefore considered a cryptic species and becomes conspicuous only when in flower during the summer months, from November to March (Figure 3C).

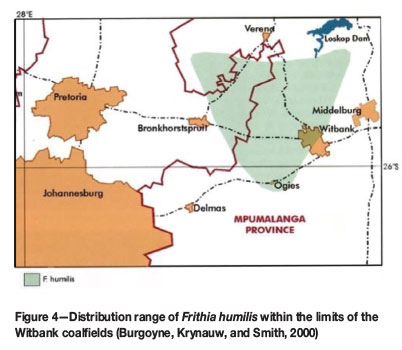
The species is restricted to the Rand Highveld grassland of Gauteng and Mpumalanga (Figure 4; Mucina and Rutherford, 2006) and is regarded as endemic to this region (Burgoyne and Krynauw, 2005). It was assessed as being Endangered (EN), due mainly to its limited distribution range (the extent of occurrence being less than 3 000 km2) and its fragmented habitat (Burgoyne and Krynauw, 2005). Populations of the species are continually declining, as suitable habitats are increasingly being threatened by expanding mining activities, informal settlements, overgrazing, alien vegetation, and unscrupulous harvesting for the horticultural trade (Burgoyne and Krynauw, 2005).
Study area
The translocation localities (receptor sites) were chosen within the Rand Highveld Grassland vegetation type (Mucina and Rutherford, 2006). Receptor sites were selected between Balmoral and eMalahleni (formerly Witbank) in Mpumalanga Province, on the periphery of the distribution range of the species. A number of potential translocation microsites were identified at each receptor site to which the rescued population was to be transplanted (Figure 5 and Table I).

The following guidelines were applied to select appropriate receptor sites and patches for translocation (Burgoyne and Hoffman, 2011):
1. F. humilis is a habitat specialist, requiring porous substrates to avoid inundation during the rainy season; Ecca and Dwyka sandstone of the Karoo Supergroup were the most suitable geological formations
2. Shallow grit-pans on exposed sandstone ridges had to be present into which the species could be transplanted
3. Intact habitat was a prerequisite, with limited ecological disruptions to prevent further disturbance of translocated populations
4. No other F. humilis populations should be present to avoid gene pool contamination
5. Sites nearest the donor site were considered ideal, as too great a distance could impair genetic variation and potential exchange
6. Conserved or protected areas are priority to avoid threats and to ensure the long-term persistence of the translocated populations
7. Minimal slope was preferred to prevent erosion and the washing out of translocated plants.
After the seven guidelines were considered at a range of potential receptor sites, which were identified during an aerial survey, the largest portion of the original donor population was translocated to a sandstone hill on private property, namely Goedvertroud (G) (Figure 5). This habitat is characterized by outcrops of the Ecca Group and was the only geologically suitable site that adhered to all the guidelines. Quartz pebbles, which facilitate seed germination (Burgoyne, Smith, and Du Plessis, 2000), were present in abundance. Topsoil layers were intact, protecting plants during the dry season. The slope was <3°.
Receptor site G is not protected by law and forms part of an abandoned coal mine with acid mine drainage along the footslopes of the hill. The mine water does not directly affect the upslope translocated populations (Burgoyne and Hoffman, 2011). Furthermore, past mining activities have resulted in major structural cracks which impede expansion of F. humilis populations due to severe water runoff. An established population of invasive Acacia mearnsii (black wattle trees) is present close to the populations, but no invasion of the receptor patches has been noticed.
Plants translocated to receptor site G were planted in patch clusters according to the availability of appropriate sandstone plates, namely GL, GM, GR, and GW. Each cluster was treated as a separate population, due to differing micro-environmental conditions (Table I) (Jusaitis, 2005). Dividing the G population in this manner also enabled more accurate statistical analyses.
Experimental translocations were also performed to test the response of the species, an edaphic specialist, to non-native geologies (Burgoyne and Hoffman, 2011). Neither of the two chosen receptor sites contained the typical geological substrate preferred by F. humilis. A part of the donor population was translocated to Eagles Rock Private Estate (E), a privately owned farm north-east of eMalahleni, and to a municipal reserve, Witbank Nature Reserve (W). The futures of the populations at these sites were more secure as receptor site E is managed by a trust and W is gazetted.
The E habitat comprises Waterberg Group sandstone, which is more compact (and less porous) than Ecca sandstones. Dominant outcrops at W were felsite rock plates from the Rooiberg Group (Transvaal Supergroup). These igneous rocks are also denser than those of typical F. humilis habitats. Both of these sites therefore posed an inundation risk during the rainy season, creating sub-optimal conditions for plant health (Burgoyne and Hoffman, 2011).
Patches of receptor site E have gentle slopes (5°) which are prone to sheet erosion. Hence, the patches are prone to topsoil and quartz pebbles being washed away, which could leave many adult plants uncovered with roots exposed (Figure 3D). One patch hostedSelaginella dregei, while another was the only other patch housing another species, namely a miniature grass, Microchloa cafa.
At receptor site W, the patches did not slope, which heightened the risk of inundation during the rainy season. Furthermore, this receptor site did not contain appropriate quartz pebbles, but larger, angular pebbles, which did not seem to provide appropriate protective conditions for germination. However, the presence of larger rocks provided protection for adult plants. Miniature succulent species, Anacampseros subnuda and Crassula setulosa, were present.
Sods of 40 x 40 x 5 (thick) cm containing plants and seed bank were lifted from the rock plates of the donor population during the translocation procedure, and transported and placed securely on rock plates at the receptor sites, creating 23 patches (Figure 5; Table I).
Post-translocation monitoring
Population censuses
An intensive post-translocation monitoring programme, by means of population censuses, was implemented over the course of three years (2010 - 2012) to gather data on trends in population sizes. Accurate and informative population data could be gathered whereby each plant was accounted for (Joseph et al., 2006).
The presence of reproductive individuals (i.e. flowering plants) and seedlings is a prerequisite to estimating potential population persistence (Mueck, 2000). Over 3000 adult individuals and seedlings, as well as the seed banks, were translocated in soil sods (Burgoyne and Hoffman, 2011). The large sample size, and the difficulty of tracking individual plants due to their small size and seasonal retraction, required a system to measure the progress of population survival, plant growth, and reproductive success.
Relative size classes
Size classes for succulents can be determined by the number of branches in case of shrubs (e.g. Euphorbia; Knowles and Witkowski, 2000), rosette diameter when studying large forbs (e.g. Aloe; Pfab and Scholes, 2004), or number of leaves where small forbs are concerned (e.g. Haworthia; Biko'o et al., 2011). Relative size classes were established for F. humilis (Table II) based on the number of leaves per plant.
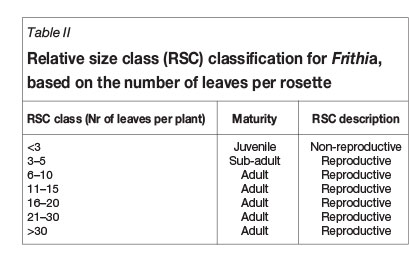
Results from ex-situ studies on the species revealed that the number of leaves is a credible indication of relative F. humilis age. These surveys showed that no individual with less than three leaves produced flowers (Orlekowsky et al., 2013). Such individuals could therefore be regarded as juveniles (non-reproductive individuals). The remaining groups comprised flower-bearing, reproductive individuals with size class 3-5 classified as the sub-adult group since its flower frequency represents a transition between juveniles and peak reproductive phases (Orlekowsky et al., 2013). All other size classes were regarded as adult (Table II).
Size classes enabled the convenient comparison of trends in the translocated populations. Hence, the stabilization or demise of populations over time could be derived and estimations of population establishment made. Monitoring data, when related to population structure (size class distribution), and thus reproductive potential, provide a valuable gauge to estimate the success of the translocation.
Total counts
The main approach with total counts is to account for every individual in a translocation patch. Such a detailed survey serves as a baseline for future comparative purposes. Comprehensive baseline data was gathered for the translocated populations and all individuals at each translocation patch were counted. Count data was converted to mean total abundance per size class per translocation patch for each receptor site.
Monitoring season
Due to the seasonal appearance of F. humilis, i.e. it being cryptic during the dry winter months (Figure 3B), the populations were monitored only during the rainy season and peak flowering time (November to February).
Data analysis
Repeated measures analysis of variance (ANOVA) was applied to the data-set to determine significant differences in relative size class abundance and flowering between sites and over time. A mixed model analysis was also performed, enabling a more accurate and robust comparison between sites over time. A mixed model considers the dependency of the data, as each population at a particular site is derived from the previous season's population (StatSoft, 2011).
Results
Monitoring began in November 2009, six months after the translocation. No comprehensive data was collected on population size at the time of translocation in 2009, and the figures recorded during the first survey were therefore considered as a baseline. The results therefore depict the initial (short-term) response (2010-2012) of the populations to translocation disturbance.
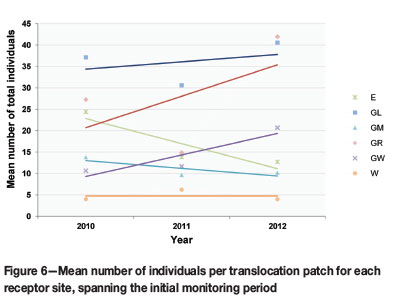
The translocated cohort as a whole has shown neither a significant decline nor increase over the course of three years, as p-values from mixed model statistical analyses ranged from 0.398 to 0.968 (a p-value of <0.05 indicates a significant difference between yearly population sizes). Despite an initial decline in the mean total cohort during 2011, the plant numbers increased during 2012. Three receptor sites (GL, GR, and GW) contributed to the growth of the translocation cohort during this period (Figure 6).
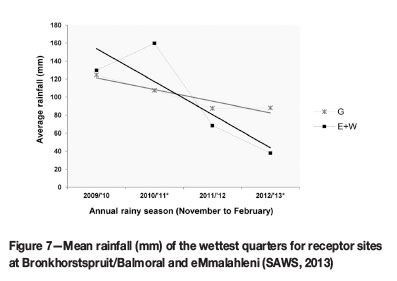
To understand population size fluctuations, rainfall trends also had to be considered. Even though these trends were not statistically analysed, patterns observed in mean seasonal precipitation can be loosely linked with patterns in population size. Overall precipitation in the study area decreased over the monitoring period (Figure 7) (SAWS, 2013). The decrease was most pronounced in the eMalahleni area, which included receptor sites E and W.
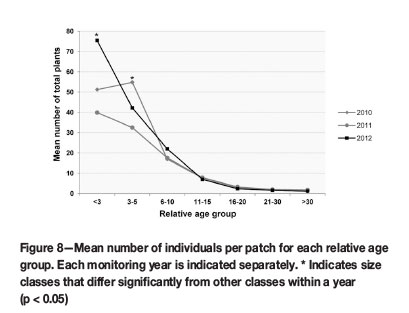
Smaller plants in size classes <3 (seedling) and 3-5 (sub-adult) contributed significantly (p <0.05) to the overall cohort population size, as the mean numbers of these groups had p-values between 0.000 and 0.001, when compared to older groups (Figure 8). The mean sizes of the seedling and sub-adult groups did not differ significantly. More importantly, these groups were the only size classes that fluctuated noticeably over time. Larger size classes were more stable in numbers (Figure 8).
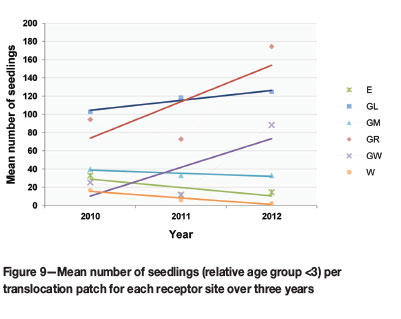
Seedling recruitment by the translocation cohort in different receptor habitats required a closer look at the seedling (size class <3) component at each receptor site. Statistical tests indicated that translocation receptor sites did influence the differences in mean seedling number (p-value 0.046). GL and GR had significantly more seedlings than the other sites. Three sites (GL, GR, and GW) also showed an increase in seedling numbers over the three years (Figure 9).
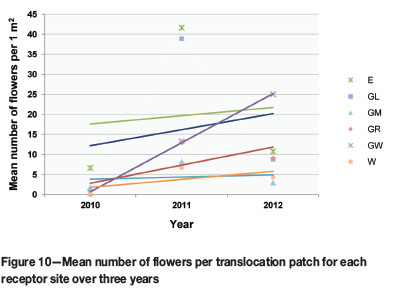
The mean number of flowers, an indication of reproductive or seed-producing potential, significantly increased per patch over the total monitoring period, across all study populations (p-value 0.000). The decrease in flowering from 2011 to 2012 was not found to be significant (p-value 0.305). A closer look at which populations flowered most abundantly revealed that populations at E and GL flowered significantly more than the other populations overall, but that GW had the steepest increase in flowering over the monitoring period (Figure 10). GR and GW showed a significant overall increase in flowering (p-value 0.022 and 0.032 respectively).
Discussion
The decline in population numbers from 2010 to 2011 (Figure 6) is an indication of the mega-disturbance caused by the translocation (Fahselt, 2007). Mortality is usually high when individuals are shifted to new environments, e.g. Mueck (2000). In the case of Frithia humilis it can be ascribed mainly to physical damage during the translocation, erosion that exposed the root systems (Figure 3D), and herbivory by rodents and insects (Figure 3E, F).
Rainfall is an important determinant in plant growth and establishment (Good et al., 1999; Jusaitis, Polomka, and Sorensen, 2004). Sites E and W experienced less rain during the wettest quarter since the 2011/2012 rainy season than did sites at G (Figure 7). The G sites showed substantial population growth from 2011 to 2012, while a reduction in E and W populations was observed over this period. This suggests that rainfall could be a vital factor in population growth and establishment during the initial years. However, flowering declined in all populations, except GW, since the 2011/2012 rainy season. This requires further investigation.
Seedlings and juveniles comprised the largest portion of the translocated populations (Figure 8). More importantly, these groups were the only size classes that increased noticeably over time. Although not statistically significant, these changes were notable, since younger groups contribute significantly to population size, as was shown for the succulent Euphorbia barnardii (Knowles and Witkowski, 2000). High numbers of seedlings and stability in the number of reproductive individuals indicated effective seedling recruitment and population persistence in such populations (Knowles and Witkowski, 2000). Similarly, Biko'o, Du Plessis, and Myburgh (2011) found in populations of Haworthia koelmaniorum var. mcmurtryi that low numbers of seedlings indicated low recruitment rates, heightening the probability of extinction in such populations.
The overall fluctuation of the F. humilis translocation cohort was more pronounced in the younger size classes (<3, 3-5, and 6-10), indicating fluctuations in seedling recruitment, while older groups remained more stable (Figure 8). The seedling group is responsible for the largest increase in population size from 2011-2012 (Figure 8), which is ascribed partially to the significant upsurge in flowering from 2010 to 2011 and possible seed production during 2011. Germination and seedling recruitment in the 2011/2012 growing season was therefore successful, in spite of the reduction in flowering over this period. Although the increase in seedling numbers (GL, GR, and GW) was not statistically significant (Figure 9), it showed that post-translocation seed from flowering translocated individuals was viable.
Fluctuations in seedling numbers also showed similarities to general rainfall trends at different sites (Figure 7 and Figure 9): overall seedling numbers at sites E and W decreased with a reduction in flowering in the 2011/2012 season (Figure 10) and a decrease in mean seasonal rainfall (Figure 7). However, seedling numbers at sites GL, GR, and GW increased, despite a reduction in flowering at GL and GR. The lower precipitation at these sites was much less pronounced than at the E and W receptor sites.
Only site GW showed an increase in flowering during the 2011/2012 season. This is the site where plants were observed to be healthiest and where microsite conditions were optimal. There was only peripheral interference from other plant species, resulting in reduced interspecific competition, and limited erosion due to a relatively level slope. The quartz pebble and top soil layers were intact, protecting plants during the dry season.
The presence of the indigenous fern ally, Selaginella dregei, on the edges of the patches might have accounted for the 2011-2012 increase in seedlings at GR, GW, and E, as well as the stability of seedling numbers at GM. Despite the slight slope at these sites, the seeds were caught in the prostrate fern surrounding the sandstone grit pans. The absence of S. dregei at W and other E patches contributed to substrate instability in rainy seasons and poor seed retention/germination at these sites. The presence of pioneer species, including the grass species Microchloa caffra, also indicated slightly deeper and more stable soil on sandstone plates, where F. humilis seedlings were able to establish more successfully. Some parts of E (E1-3), as well as the W habitat, had noticeably less co-occurring species, shallower soils, and conspicuous water runoff areas.
Conclusions
Fluctuations in numbers have thus far not been significantly detrimental to the translocated population, since flowering was abundant and seedling recruitment ongoing. Seedlings contributed significantly to population size and thus ensured the persistence of translocated F. humilis plants at the receptor sites from season to season. Fluctuations in population numbers will continue for some years until the population has settled.
Furthermore, the overall increase in seedling numbers points to appropriate germination conditions at many receptor sites, especially those at G, the receptor sites comprising typical F. humilis geological substrate. Ideal conditions include the abiotic micro-environment (level slope, sufficient drainage, and soil stabilizing species). Our findings suggest that receptor sites with similar geological conditions to the donor site must receive priority during the translocation of an endangered habitat specialist.
In-situ conservation obviously remains the most effective and safest way of preserving endangered species. However, in the face of human development impinging on natural habitats, research such as this study that clarifies the response of such species to translocation (which may become inevitable in many cases) may prove invaluable for the design of future translocation procedures and monitoring strategies. The results of this study serve as a baseline for future studies at the receptor sites. Ongoing monitoring will endeavour to determine whether translocation was a successful mitigation measure. Even if the future outcome is negative, this study would have contributed to our knowledge of translocation ecology in South Africa.
General recommendations
With the probability of translocation becoming an increasingly important environmental mitigation tool in the mining sector, along with the lack of peer-reviewed literature on translocation in South Africa (Milton et al., 1999), scientifically sound research on translocation procedures and their effects is required. Furthermore, no reference is made to translocation for conservation purposes in South African legislation regarding endangered wildlife species (National Environmental Management: Biodiversity Act, 10 of 2004). Hence, translocation is very rarely considered as a mitigation measure when species are threatened by local extinctions.
Insights gleaned from this study will inform mining institutions faced with similar conservation challenges. Firstly, comprehensive knowledge of the target species, its habitat requirements, and ecological interactions is needed when a translocation protocol for a specific species is designed (Gordon, 1996; Parsons and Zedler, 1997).The participation of conservation biologists is therefore imperative.
Secondly, a long-term monitoring programme has to be initiated. Information gathered from post-translocation monitoring is the cornerstone of reliable knowledge on species translocation (Menges, 2008). Clear research questions, scientifically robust monitoring methodology, and appropriate sample size whereby statistically practical data sampling can be employed are pivotal to acquiring data for meaningful analyses. Volunteer organizations such as CREW (Custodians of Rare and Endangered Wildflowers)1 should be involved in the monitoring and to encourage public participation.
The above-mentioned recommendations are well within the capacity of mining companies, as proved by Inyanda Coal and Exxaro. Mining companies can vastly improve our knowledge of rare or endangered species translocation in South Africa by involving relevant expertise, research institutes, and volunteer organizations (Milton et al., 1999). Properly recorded, documented, analysed, and published monitoring data can regularly advise future policies on species translocations.
References
Biko'o, A.A., Du Plessis, D.G.C., and Myburgh, W.J. 2011. Population size, structure and habitat features of Haworthia koelmaniorum var. mcmurtryi, an endemic plant from Mpumalanga Province, South Africa. Koedoe, vol. 53, no.1. pp.1-8. [ Links ]
Burgoyne, P., Krynauw, S., and Smith, G. 2000. Frithia - up close and personal. Aloe, vol. 37, no. 2/3. pp. 38-42. [ Links ]
Burgoyne, P.M., Smith, G., and Du Plessis, F. 2000. Notes on the genus Frithia (Mesembryanthemaceae) and the description of a new species, F. humilis. Bothalia, vol. 30, no. 1. pp. 1-7. [ Links ]
Burgoyne, P.M. and Hoffman, A. 2011. Frithia humilis - notes on the translo-cation of a Red Listed succulent. Aloe, vol. 48, no. 2. pp. 38-40. [ Links ]
Burgoyne, P.M. and Krynauw, S. 2005. Frithia humilis Burgoyne. National Assessment: Red List of South African Plants version 2013.1. Raimondo, D., Von Staden, L., Foden, W., Victor, J.E., Helme, N.A., Turner, R.C., Kamundi, D.A. and Manyama, P.A. (eds.). Strelitzia, vol. 25. South African National Biodiversity Institute, Pretoria. pp. 423. [ Links ]
Cairncross, B. 2001. An overview of the Permian (Karoo) coal deposits of southern Africa. Journal of African Earth Sciences, vol. 33, no, 3-4. pp. 529-562. [ Links ]
Fahselt, D.F.D. 2007. Is transplanting an effective means of preserving vegetation? Canadian Journal of Botany, vol. 85, no. 10. pp. 1007-1017. [ Links ]
Good, J., Wallace, H., Stevens, P., and Radford, G. 1999. Translocation of herb rich grassland from a site in Wales prior to opencast coal extraction. Restoration Ecology, vol. 7, no. 4. pp. 336-347. [ Links ]
Gordon, D. 1994. Translocation of species into conservation areas: a key for natural resource managers. Natural Areas Journal, vol. 14, no. 1. pp. 31-37. [ Links ]
Gordon, D.R. 1996. Experimental translocation of the endangered shrub Apalachicola rosemary Conradina glabra to the Apalachicola Bluffs and Ravines Preserve, Florida. Biological Conservation, vol. 77, no. 1. pp. 19-26. [ Links ]
Griffith, B., Scott, J.M., Carpenter, J.W., and Reed, C. 1989. Translocation as a species conservation tool: status and strategy. Science, vol. 245, vol. 4917. pp. 477-480. [ Links ]
IUCN (World Conservation Union). 1987. IUCN position statement on translo-cation of living organisms. Introductions, re-introductions, and restocking. IUCN, Gland, Switzerland. [ Links ]
IUCN (World Conservation Union). 1998. IUCN guidelines for re-introductions., Gland, Switzerland and Cambridge. [ Links ]
Joseph, L.N., Field, S.A., Wilcox, C., and Possingham, H.P. 2006. Presence-absence versus abundance data for monitoring threatened species. Conservation Biology, vol. 20, no. 6. pp. 1679-1687. [ Links ]
JUSAITIS, M. 2005. Translocation trials confirm specific factors affecting the establishment of three endangered plant species. Ecological Management and Restoration, vol 6, no. 1. pp. 61-67. [ Links ]
Jusaitis, M., Polomka, L., and Sorensen, B. 2004. Habitat specificity, seed germination and experimental translocation of the endangered herb Brachycome muelleri (Asteraceae). Biological Conservation, vol. 116, no. 2. pp. 251-266. [ Links ]
Knowles, L. and Witkowski, E.T.F. 2000. Conservation biology of the succulent shrub, Euphorbia barnardii, a serpentine endemic of the Northern Province, South Africa. Austral Ecology, vol. 25, no. 3. pp. 241-252. [ Links ]
MCCLELAND, W. 2009. Survey of the Frithia humilis Burgoyne population on the Inyanda Coal Mine Property, Witbank (Mpumalanga). Final report. De Castro, T. (ed.). De Castro and Brits Ecological Consultants. Cresta, Randburg. 8 pp. [ Links ]
MENGES, E.S. 2008. Restoration demography and genetics of plants: when is a translocation successful? Australian Journal of Botany, vol. 56, no. 3. pp. 187-196. [ Links ]
Milton, S., Bond, W., Du Plessis, M., Gibbs, D., Hilton Taylor, C., Linder, H., Raitt, L., Wood, J., and Donaldson, J. 1999. A protocol for plant conservation by translocation in threatened lowland Fynbos. Conservation Biology, vol. 13, no. 4. pp. 735-743. [ Links ]
Moore, R. 1986. Nodes from the underground. Natural History, vol. 95, vol. 10. pp. 64-67. [ Links ]
Mucina, L. and Rutherford, M.C. 2006. The vegetation of South Africa, Lesotho and Swaziland. Strelitzia, vol. 19. South African National Biodiversity institute, Pretoria. [ Links ]
Mueck, B.S.G. 2000. Translocation of Plains Rice flower (Pimelea spinescens ssp. spinescens), Laverton, Victoria. Ecological Management and Restoration, vol. 1, no. 2. pp. 111-116. [ Links ]
Orlekowsky, T., Viviers, J., Kruger, E., and Siebert, S.J. 2013. Ex situ monitering van Frithia humilis Burgcoyne: 'n Kontrole studie vir 'n translokasie projek. Suid-Afrikaanse Tydskrif vir Natuurwetenskap en Tegnologie, vol. 32, no. 1. [ Links ]
Parsons, L.S. and Zedler, J.B. 1997. Factors affecting reestablishment of an endangered annual plant at a California salt marsh. Ecological Applications, vol. 7, no. 1. pp. 253-267. [ Links ]
Pfab, M.F. and Scholes, M.A. 2004. Is the collection of Aloe peglerae from the wild sustainable? An evaluation using stochastic population modelling. Biological Conservation, vol. 118, no. 5. pp. 695-701. [ Links ]
Seddon, P.J. 2010. From reintroduction to assisted colonization: moving along the conservation translocation spectrum. Restoration Ecology, vol. 18, no. 6. pp. 769-802. [ Links ]
StatSoft. 2011. STATISTICA (Data analysis software system). [ Links ] ♦
1 More details about this organization can be found at http://www.sanbi.org/programmes/threats/crew
© The Southern African Institute of Mining and Metallurgy, 2014. ISSN2225-6253. This paper was first presented at the, Mining, Environment and Society Conference 2013, 27-28 November 2013, Misty Hills Country Hotel and Conference Centre, Cradle of Humankind, Muldersdrft.














