Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.114 n.6 Johannesburg Jun. 2014
GENERAL PAPERS
Dealing with open fire in an underground coal mine by ventilation control techniques
by N. SahayI; A. SinhaI; B. HaribabuII; P.K. RoychoudharyII
IMine Ventilation Discipline CIMFR, Dhanbad (Jharkhand), India
IISECL, Bilaspur, India
SYNOPSIS
Open fire in coal mines is one of the most serious threats to miners, as well as to the mine. Open fire can often be effectively dealt with by prompt local action, otherwise it very quickly becomes uncontrollable. In one incident, none of the available open fire control technologies, viz., water deluge and sprinkler systems, high-expansion foam, high-pressure high-stability nitrogen foam, water misting, and ventilation and pressure control techniques, were effective for saving the mine without sealing from surface, since the fuel-rich environment prohibited underground access due to the methane explosion hazard.
The authors have developed a methodology for dealing with advanced-stage open fires underground by the application of a modified ventilation control technique. It is based primarily on a better understanding of the behaviour of open fires, proper diagnosis of the problem, application of judicious ventilation control techniques, and selection of suitable fire indices for assessing the status of an open fire. This methodology was used to successfully control an open fire in Surakachhar 3 and 4 incline mine Surakachhar, central India. The fire area was sealed underground and production subsequently resumed in record time. The paper discusses the behaviour of open fires, particulars of the mine, diagnosis of the problem, experimentation methods, and the results obtained.
Keywords: spontaneous combustion, high-pressure hig-stability nitrogen foam, water misting technology, ventilation and pressure control techniques, oxygen-rich and fuel-rich fire.
Introduction
Occurrence of open fire in underground coal mines is one of the most feared hazards throughout the world. During the 18th and 19th centuries, enormous losses of life and property occurred due to mine fires and explosions. Mine fires are mainly caused by sluggish ventilation, frictional heat, electrical sparks, or spontaneous combustion in coal heaps lying in airways that form a part of the active ventilation system. A mine fire can pollute the entire underground atmosphere in a very short time, resulting in loss of life and sometimes closure of the operation. Recent occurrences of open fire in Indian coal mines are Noonidih-Jitpur mine, M/s Steel Authority of India Limited, Burnpur, (2007), Kunustoria colliery, M/s Eastern Coalfields Limited, Sunctoria (2009), Majiri mine No. 3, M/s Western Coalfields Limited, (WCL), Nagpur (2010), Anjan Hill mine, (2010), Bartunga mine (2010), and 3 & 4 Incline mine Surakachhar (2011), M/s South Eastern Coalfields Limited, Bilaspur, 3 & 4 Pits Ballarpur colliery, WCL(2012), Nagpur. The efficacy of the measures and technologies for controlling open fire are mostly dependent on the location, status, and duration of the fire. intensity and duration are also dominating factors for the better efficacy of the measures. Effective techniques require a better understating of the strengths and limitations of each measure and the behaviour of the fire, and a realistic work programme based on the extent and rate of progress of the fire.
Methods for controlling open fires
The techniques and methodologies for controlling open fire may be broadly divided in two groups, viz., direct and indirect methods.
Direct methods
The methods for direct fighting of open fire, such as water deluge and sprinkler systems (McPherson, 1993a, ch. 21), can be very effective in areas close to fixed equipment and activated by thermal sensors. A major difficulty in subsurface firefighting is the limited reach of water jets imposed by the height of the airway. It has been projected that a line pressure of the order of 800-1400 kPa would be required to increase the effective range of a typical water spray to 30 m.
High-expansion foam (McPherson, 1993b) containing large volumes of water-based foam can provide a valuable tool for fighting fires in enclosed spaces such as a single blind gallery. The bubbles are generated by a fan that blows air through a fabric net stretched across the diffuser. The net is sprayed continuously with a mixture of water and foaming agent. Ammonium lauryl sulphate foaming agent mixed with carboxyle-methyl-cellulose improves the stability of the bubbles. A major limitation is obstructions caused by roof falls, which are quite liable to occur during fighting of a large underground fire. A foam plug may not be able to climb over such obstructions with the available ventilating pressure.
High-pressure high-stability nitrogen foam (HPHS) (Voracek, 1994) with cooling, inertizing, and inhibiting properties is effective when applied upstream of the fire location under a controlled air flow rate. HPHS nitrogen foam in conjunction with the chamber method of ventilation was applied for successful control of an open fire in the goaf of a longwall panel at 1&2 Incline mine Jhanjra, ECL (Sahay et al., 2001) under the aegis Coal India Limited, Kolkata, India. Another underground open fire close to a downcast shaft in one of the intake airway at Noonidih-Jitpur colliery (Sahay et al., 2008) was also controlled by application of HPHS nitrogen foam in conjunction with regulation of the ventilation control to avoid methane accumulation.
Water misting technology (Liu and Kim, 2000), based on wetting of air flowing to the seat of fire through adding water droplets of the order of 400 µM or less in size to extract heat directly from the seat of the fire by evaporation with the creation of an oxygen-deficient environment near the seat of the fire, is an added advantage. Water mist does not behave like a true gaseous agent in fire suppression. The effectiveness of a water misting system is dependent on spray characteristics like the size distribution of the droplets, flux density, and spray dynamics with respect to the fire scenario. Due to the complex extinguishing process the relationship between a fire scenario and the characteristic of a water misting system is not understood well enough to design a compatible water misting system. The efficacy of these technologies is location- as well as capacity-dependent. The water misting technology has yet to be applied in Indian coal mines, although its efficacy was found to be better than that of HPHS nitrogen foam when it was applied from the close vicinity of a fire during an experiment in a mine fire model gallery (MFMG) (Singh et al., 2007).
Indirect methods
Ventilation control techniques (McPherson, 2003a, ch. 21) have three types of effect on fire: (i) the combustion process and distribution of products of combustion, (ii) direction and rate of propagation of the fire, and (iii) air flow distributions in other parts of the mine. In controlling of air flow rate in an open ventilation circuit, the probability of shifting the environment of the fire area from oxygen-rich to fuel-rich is increased due to less dissipation of convective heat. Moreover, the status of an open fire is very sensitive to air velocity. Ventilation control techniques therefore required to be applied judiciously in the case of a complex ventilation network.
In the pressure control technique (Kissell and Timko1991), airways parallel and adjacent to the fire path are kept free from fire gases by maintaining higher atmospheric pressure with the help of brattice stopping erected in crosscuts to facilitate the application of water sprays into the fire path. Devices such as the 'parachute stopping' or 'inflatable seal' have been developed to replace brattice cloths in such circumstances. These can be erected quickly and give improved seals around the perimeter of the airway. A consequence of this technique is that the air flow over the fire is increased to an extent that depends upon the configuration and resistances of the local airways. Pressure differentials between airways can also be modified by the use of a temporary fan instead of a restriction in the adjacent airway. In this case, air flow over the fire will be reduced. The location of and pressure developed by the fan is required to be selected with care in order to avoid aggravation of the fire.
In many cases fire is located at a remote place in the mine and is detected only at the advanced stage, while main return airways become badly polluted with hazardous gases. This necessitates suspending the underground deployment of personnel due to the methane explosion hazard. In this situation two options are adopted. One option is sealing of the mine entries from surface followed by application of measures for controlling the fire followed by recovery of the mine, Moreover, the danger of methane explosion is always associated with the simultaneous sealing upstream and downstream or only downstream of an open fire, particularly in a fuel-rich environment. Methane explosions have occurred (Urosek et al., n.d.) 30 hours and 77 hours after sealing of open fires in longwall panels in coal mines at Delta Colorado and Morgantown, West Virginia respectively. At the same time, sealing downstream of an open fire is difficult and risky due to the hot and hazardous environment that prevails after sealing upstream of the fire location. Hence sealing of the area affected by open underground fire by conventional approaches is a risky proposition. Conversely, sealing of the mine from the surface is time-consuming and costly, causing suspension of production for a long period. Sometimes the mine, once sealed off due to fire, has to face problems of waterlogging and deterioration of roof conditions.
In the light of the above, to overcome the constraints and limitations of various open fire control techniques, the authors have developed a methodology for dealing with open fire in critical situations and the safe isolation of the fire-affected area by a modified ventilation-control technique. The sequence of measures is sealing at a strategic location upstream of the fire to short-circuit the air flow adjacent to the sealing, stagewise reduction of fan pressure and barometric pressure over the fire while closely monitoring the status of the fire, followed by sealing of the fire area underground. This technique was applied for controlling an open fire at 3 and 4 Inclines Mine Surakachhar, (SECL), Bilaspur, situated in central India. The behaviour of the fire, particulars of the mine, and diagnosis of the problem, experimentation, and results are discussed in subsequent sections.
Behaviour of underground fires
Fires in underground coal mines are classified broadly into two groups, viz. open and concealed fires (McPherson, 1993b). Open fires are accompanied by flaming combustion because of the availability of sufficient oxygen in the ventilation circuit. Conversely, concealed fires occur in areas that are inaccessible such as caved goaf or abandoned zones or sealed-off area. A closed fire can also become an open fire after crossing over the seals/stoppings. The behaviour of open fire depends on factors such as the nature and amount of flammable material, ventilation system arrangement, the duration of the fire, the extent of the spread of combustion products, and ignition location. During initiation of an open fire the available oxygen is utilized in the formation of principally carbon dioxide and carbon monoxide. The chemical reactions that take place in the oxidation zone are (Pandey et al., 2003):

All these reactions are exothermic, and raise the temperature of the coal to a high value. In this situation, the air temperature downstream of the fire increases due to the convective and radiative heat carried by the air current.
Depleted of free oxygen, the fire situation moves into the reduction zone, where CO2 is reduced to CO in contact with hot coal. Where water is present in the fire zone, steam is produced, which reacts with the hot coal and produces additional combustibles gases and frequently water gas. The chemical reactions in this zone are believed to be:
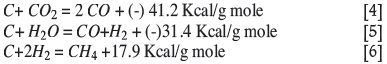
The heat generated during reactions [1]-[3] in the oxidation zone is neutralized by endothermic reactions [4] and [5] in the reduction zone. Hence the area downstream of the fire zone initially contain hot combustibles and is deficient in oxygen. The progress of the reaction from oxidation to reduction may be identified by the increasing trend of CO, H2, and CH4 in air samples, followed by a decreasing temperature trend.
Effect of ventilation on open fire
The effect of ventilation on open fire is a complex phenomenon. Initially, ventilation is influenced by an open fire. An open fire causes a sharp increase in the temperature, resulting in expansion of the air and the addition of combustion products and evaporated water (Gillies, Wu, and Humphreys (2004). The expanded air moves in both directions along the airway, which reduces the air flow rate in an upstream direction and increases the air velocity downstream of fire location, causing an additional pressure loss. This is known as the choking or throttling effect. The choking effect is analogous to increasing the resistance of the airway, and is increased in the case of a single airway. The immediate effect of heat on the ventilating air stream is very localized (Litton, Derosa, and Li, 1987). The reduced density causes the mixture of hot air and combustion products to rise and flow preferentially along the roof of the airway, and under low air velocity in a level or descending airway they back up against the direction of air flow. In this way the air flow to the fire and discharge of fire gases become bi-directional. This situation may also prevail in case of choking upstream of the fire location. Similar results have also been observed during experiments (CIMFR, 2004; Singh et al., 2004) carried out in a 65.5 m mine fire model gallery (MFMG) at the Central Institute of Mining and Fuel Research, Dhanbad, India. This facility, which is equipped with state-of-the-art instrumentation to study the behaviour of open fires at 30-second intervals, is used to assess the efficacy of fire control measures. The experiment was conducted with 10 cm thick coal slab layered on the inner wall of an arched-shaped gallery of cross section 5.78 m2 up to 22.0 m by establishing an air velocity of 1 m/s with the help of axial flow fan (AF-50) installed at the other end of the gallery. Photographs of the experiment are shown in Figure 1.
Initially, the air flow was unidirectional (Figure. 1a). During the experiment it was observed that the lower portion of the gallery (from the floor to a height of about 1.4 m) from entry to the fire location was acting as intake air, while upper portion of the gallery was acting as a return (Figure 1b), despite the fan in operation, after about two hours from the initiation of the fire. It was also observed that hot gases containing coal dust started burning at the entry (Figure 1c). Temperatures in the fire zone between 800°C and 1058°C were recorded during the experiment. A similar phenomenon may occur in a mine. This phenomenon is more probable in the case of a single or blind gallery. The availability of oxygen at the fire site regulates the development of the fire and the shifting of the environment from oxygen-rich to fuel-rich, as indicated by a sharp decrease in oxygen percentage and rise in concentration of CO, CH4, and H2 in air samples. In case of fire in a fuel-rich environment the value of the Jones and Tricket ratio (JTR) lies between 1 and 1.5 (Banerjee, 2000). This is a serious progression and makes it difficult for firefighters to take proper decisions.
Diagnosis of problem and experimentation
Surakachhar 3 & 4 Incline Mine belongs to M/s SECL, and is situated in Bilaspur in the Korba district of Chhattisgarh State in central India. The mine produces approximately 500 t of coal per day and deploys 1200 persons in the largest shift.
Particulars of the mine
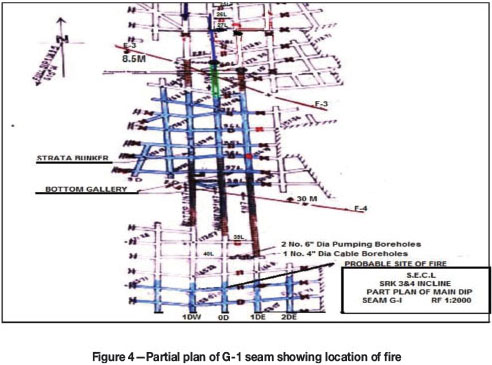
In the mine there are two seams viz., G-1 and G-3. The lowermost seam is G-1, which is 1.5-3.0 m thick and has a gradient of 1:9.5, dipping S-15°W, with reserves of 2.554 Mt. The mine has been developed by the bord and pillar method along the G-1 seam, at depths from 46 m to 240 m. The extent of development in N-S direction along dip is up to 72L and in an E-W direction along strike is from 3D up to the mine boundary. Gassiness of the seam is Degree-II as per Coal Mines Regulations-1957, India.
The G-1 seam (Figure 4) is accessed from surface through two inclines. viz., Inclines 3 and 4, and an air shaft. The No. 3 incline connected with 0D and No.4 connected with 1DW are equipped with conveyor belt and haulage respectively. There are two major faults on the south side of the mining area. A downthrow fault F3-F3 with 8.5 m displacement intersects the seam between 29L and 32L and an upthrow fault F4-F4 with 30 m displacement intersects the seam between 38L and 39L. These faults thus create a natural basin formation of the seam between 29L and 39L. The airways, viz., 0D, 1DW, and 1DE, on both sides of faulted zone are connected through drifts. The roof of the seam consists of alternating shale and sandstone whereas the floor consists of shale and coal. The immediate roof of the airway consists of a coal layer about 0.5 m-0.8 m in thickness. There is one depillaring district (W-5B) in the west side of the mine and a pumping station on 62L in 0D. On the downdip side there are two boreholes (depth 116 m, diameter 100 mm) at 40L/1DE drilled from surface. One borehole is for laying electrical cables and another for discharging of underground water. The mine ventilation is via an exhaust system, achieved by an axial flow fan (SIWAX-APG-2159) of capacity 67 m3/s at a pressure of 650 Pa installed on the surface and connected with the air shaft through a fandrift. There is another fandrift connected with the same airshaft with proper locking arrangements. Prior to the fire, intake air entered the mine through Inclines 3 and 4 and flowed along 0D and 1DW up to 25L, where it was split in two streams. The majority of the intake air (about 45.0 m3/s) was directed along 25L and 26L to ventilate the depillaring district (W-5B), while a more restricted part of the intake air (about 11 m3/s) flowed through 0D and 1DW to ventilate the pumping station. To control air flow rate on the dip side below 25L a regulator was installed on 62L beween 0D and 1DE. The return air from the pumping station flowed through a single airway 1DE. In the ventilation circuit doors at 12L and 27L between 0D and 1DE are provided. Other details are shown in schematic diagram of the ventilation network of the mine (Figure 2)
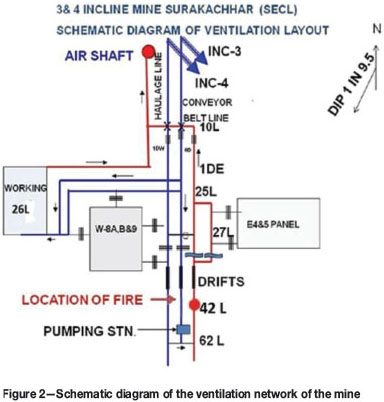
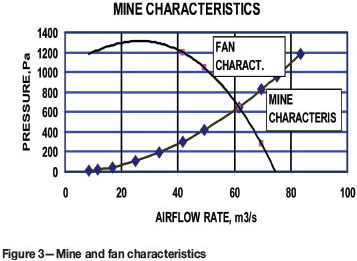
A resistance of the mine in SI unit using Atkinson's equation was calculated of the order of 146.25 Ns2/m8. The fan and mine characteristics are depicted in Figure 3.
History of the fire
The open fire occurred at about 1.5 km from main mine entries, in 0D of G-1 seam (Intake airways) between 41L and 42L on 8 September 2011 at 1:00 a.m. The fire progressed very rapidly The intensity of the fire can be judged from the fact that the values of Graham's ratio and oxides of carbon ratio had reached levels of 28.2% and 17.7% respectively. During efforts to locate the fire the rescue team could not proceed below 28L along 0D and 1DE due to the hot and hazardous environment and poor visibility. In this situation attempts were made to isolate the fire from the main ventilation circuit below 27L by erecting brick stoppings at 0D, 1DW, 1DE, and 2DE between 27L and 28L. Two stoppings, at 0D and 1DW between 27L and 28L upstream of the fire location (intake), were completed. Downstream of the fire location (return airways) partial CGI sheet and sandbag stoppings at 1DE between 27L and 28L could be not erected due to the unbearably hot and hazardous atmosphere at the proposed location. Similarly, the proposed location for erection of stopping at 2DE could not be approached. On 10 September 2011, doors at 27L between 0D and 1DE were partially opened and personnel evacuated from underground. These measures did not yield the desired results, as indicated by continued discharge of CO at a concentration of about 110 ppm through the fan drift. Sealing of the mine from surface at the main entries was inevitable due to apprehension of the damage potential of such an unsealed fire. At this juncture, experimentation was carried out by the authors for controlling the fire and safely isolating the fire area underground so that early resumption of coal production would be possible. Initially, the behaviour of the fire was studied as discussed in subsequent paragraphs.
Influence of barometric pressure on fire behaviour
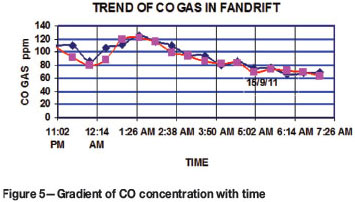
The behaviour of the fire was studied by measuring the CO gas content in air discharged through the fan drift on 14 and 15 September 2011. The results are represented in Figure 5. The results indicate minimum CO levels between 11:00 pm and 12:15 am when the barometric pressure was maximum, and maximum CO levels during minimum barometric pressure. Hence the fire was responding to diurnal changes in barometric pressure. It was inferred that the life of the fire could be prolonged by the feeding of air due to the diurnal change in barometric pressure.
Identification of chemical reaction zone
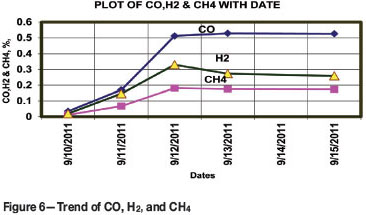
Air samples were collected through the borehole at 1DE/40L and analysed using a gas chromatograph. The results are presented in Table I. The trends of combustibles generated during the period 10-15 September 2011 were examined. The CO, H2, and CH4 trends during that period are shown graphically in Figure 6. The CO, H2, and CH4 levels rose abruptly after partial isolation of the fire area during the period between 10 and 12 September, and then levelled off. From this it may be inferred that chemical reaction was taking place in the reduction zone (see Equations [1] and [4-6]. The fire was thus in an oxygen-rich environment as the minimum O2 level was 13.3%. It was apprehended that the presence of water, heat, and coal could prolong the fire.
Assessment of status of fire
The values of the fire indices (Singh et al., 2004) viz., Graham's ratio, oxides of carbon ratio, and Jones and Tricket ratio calculated from the air sample analyses on 15 September were of the order of 6.67%, 10.88%, and 0.66 respectively. As per the index values of Graham's ratio and oxides of carbon ratio, the status of fire was considered active.
Summary of investigation and observations
The results of the investigation and observation revealed that:
1. Mine water gauge was of the order of 650 Pa
2. Air circulation into the mine was of the order of 56 m3/s
3. Double doors at 27L between 3D and 1DE were partially opened providing a parallel airway path to fire area
4. Suction pressure at the seat of fire measured through the borehole against the atmosphere was of the order of 300 Pa.
5. Gas analysis results from fan drift indicated that the fire was responding to diurnal variation in barometric pressure
6. A concentration of CO of the order of 110 ppm was measured at the fan discharge point
7. After erection of stoppings in intake airways (0D and 1DW), the direction of air flow in 1DE and 2DE was found to be bi-directional between 27L and the fire location. The return airway (1DE) alone was acting both for feeding and discharging of air from the fire area
8. The chemical reaction may be considered as being partially in the reduction zone.
Hence it was concluded that after partial sealing, the fire was active in the reduction zone under the influence of barometric pressure and fan pressure. Feeding of air to the fire was possible either through bi-directional flow of air and discharge of gases from the fire in 1DE or leakage of air through the stoppings in 3D and 4D between 27L and 28L.
Design of ventilation parameters
To control feeding of air to the fire, the optimum fan pressure was found to be in the order of 280 Pa to establish an air velocity below 0.1 m/s at 27L near fire area and 0.5 m/s in other part of the mine so as to avoid accumulation of smoke and foul gases in the main intake airways. For this purpose, the ventilation circuits of the mine were simulated using 'Vent' computer software, and taking the main branches viz. ventilation circuit from surface (INC-3)-0D and 1DW/25L-W-5 panel-27L-across door -1DE(27L-10L)- main return to fan drift) and fan system, including the surface leakage path. The results of the simulation studies further indicated that by increasing surface leakage to the order of 65.0 m3/s at the fan, the negative pressure over the fire created by the fan would be reduced to 10 Pa. In this case the fan pressure and air circulation in the mine would be of the order of 300 Pa and 11.0 m3/s respectively Furthermore, erection of a brattice curtain absorbing a pressure of 20 Pa at mouth of INC-3 during the night would reduce the effect of diurnal variation in barometric pressure over the fire by 17 Pa.
Measures taken
Accordingly, fan pressure was reduced from 650 Pa to 400 Pa in the evening of 15 September, then gradually reduced to 280 Pa in steps of 20 Pa a day from 17-22 September by opening airlock in the fan drift of the standby fan. Simultaneously, the effect of the diurnal variation in barometric pressure was neutralized by increasing the mine water gauge by 20 Pa during the night. During this process, generation and burning of methane gas in air samples collected through the borehole were monitored to maintain an oxygen-rich environment in the fire area before reducing the fan pressure. Result of analyses, using gas chromatography, of air samples collected through the borehole during the period 16-28 September are also given in Table I.
Monitoring of status of fire
In the case of an open fire, indices like Graham's ratio [CO/AO2] and oxides of carbon ratio [CO/CO2] are important, as these are independent of dilution by fresh air subject to variations in carbon monoxide and carbon dioxide that are not caused by the fire. The Jones-Tricket ratio is also considered as a measure of reliability of sample analysis as well an indicator of the type of fuel involved. The trend of saturated hydrocarbons, particularly ethylene, is a useful indicator to assess the heating of coal at low temperature or the combustion of fresh coal. Trending of CO, CH4, H2, and O2 may provide information about whether the environment is oxygen-rich or fuel-rich. The temperature at the seat of the fire could not be measured due to electrical cables laid in the borehole. The status of the fire and environment, including the efficacy of the measures taken, were assessed on the basis of analyses of air samples collected through the borehole at 1DE/40L during the period 10-28 September 2011 (Table I).
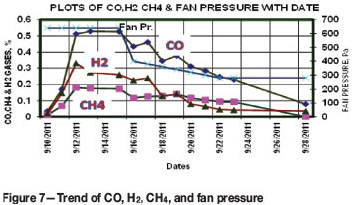
Trending of combustibles
A close vigil was kept on the status of the fire to optimize the extent and duration of the measures taken. For this purpose, combustibles gases viz. CO, CH4, and H2, from the results of analysis of air samples (Table I) were trended graphically as shown in Figure 7. The results reveal that during the period 10-15 September the values of CO, CH4, and H2 increased sharply and then leveled-off at 0.5259%, 0.1762%, and 0.259% respectively. The results also reveal that after starting the reduction in fan pressure on 15 September the values of CO, CH4, and H2 decreased sharply. At about 280 Pa of fan pressure the decreasing trend of combustibles in the air samples continued even without further reduction in fan pressure. The values of CO, CH4, and H2 in air samples collected on 28 September were of the order of 0.0786%, zero, and 0.0352% respectively.
Assessment of status of fire using Graham's ratio
Graham's ratio (Ray et al., 2004) [CO/ΔO2] is an important tool for assessing the status of an open fire. It is favored as it is independent of dilution by fresh air. It defines different stages of fire from initiation of spontaneous combustion to the blazing stage. An index value of less than 1.0 indicates a fire in the smouldering state and an index of more than 2.0 a blazing state. Trending of this ratio is essential when dealing with an open fire, and may give information regarding the progress of the fire. The values of the index were calculated from air samples taken during the period 10-28 September using the formula:
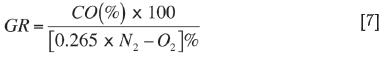
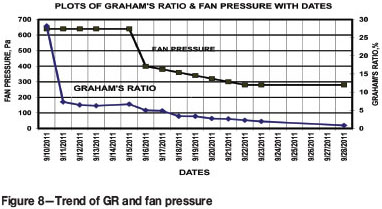
Trends of GR and fan pressure are presented in Figure 8. The results reveal a sharp decrease in index value from 28.21% to 6.45% during the period 10-12 September, followed by a levelling-off to 6.45-6.67% during the period 12-15 September. This may be due to sealing upstream of the fire. Furthermore, GR value followed a decreasing trend during the period 16-28 September and reached to a level of 0.8%, indicating a smouldering state. This may be due to the measures taken.
Assessment of status of fire using oxides of carbon ratio
The oxides of carbon ratio (IOC) [CO/CO2] (Banerjee, 2000) is also important and favoured for assessment of the status of a fire. The IOC value is also independent of dilution by fresh air or even by nitrogen. The status of the fire on the basis of this ratio is interpreted as: less than 2.0, no fire; between 2.0 and 13.0, superficial state of fire; and more than 13.0, blazing fire. This ratio, together with Graham's ratio, can be a better indicator in assessment of the status of an open fire. The IOC value of air samples (Table I) was calculated using

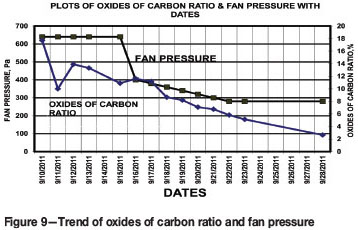
The trends of IOC and fan pressure are presented in Figure 9. The results reveal a consistent decreasing trend during the period 16-28 September, when the IOC ratio reached a level of 2.63%, indicating a superficial state of the fire.
Assessment of status of fire using Jones-Tricket ratio
The Jones-Tricket ratio (Banerjee, 2000) is used as a measure of the reliability of sample analysis, and also as an indicator of the type of fuel involved in the fire. This ratio (JTR) is also unaffected by inflows of air, methane, or injected nitrogen. It can be used for the assessment of gaseous products in case of fires and explosions. This is a useful pointer to the progression of the fire, rising during the early stages and tending to remain constant during flaming combustion. However, the JTR rises rapidly again to between 1.0 and 1.5 as the fire becomes fuel-rich. In certain types of combustion with 50% conversion from CO to CO2, the JTR may increase to 7 or more. The value of this ratio in the case of <17.0% oxygen in air samples collected from the fire area through boreholes was 0.65 to 0.85. The JTR was calculated from the air sample analysis results (Table I) using the formula
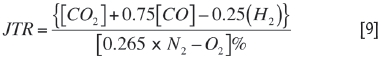
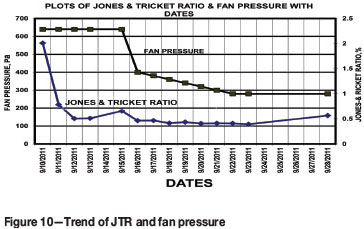
The results and fan pressures are presented in Figure.10. The results reveal that the JTR was of the order of 2.0 on 10 September, indicating a fuel-rich environment. This may indicate fuel-rich combustion conversion of CO to CO2 in the very active stage of the fire. A consistently decreasing trend can be seen during the period from 16-28 September, with the JTR reaching a level of 0.57 indicating an oxygen-rich state of the fire.
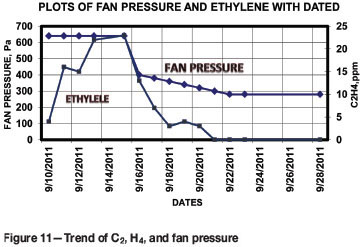
Assessment of status of fire by trending of ethylene
Previous work carried out by the Bureau (Xie et al., 2011) has shown that the desorption of low molecular weight hydrocarbon gases from coal is strongly temperature-dependent. Changes in the concentration of desorbed hydrocarbons can be detected at temperatures even less than 100°C. This may also indicate the involvement of fresh coal in the fire or the fuelling of the fire by coal detached from the sidewalls and roof. The ethylene levels from air samples (Table I) during the period 10-28 September are presented in Figure 11, together with the fan pressure. An increasing trend of ethylene is evident during the period 10-15 September, followed by a decreasing trend from 16-23 September, and finally zero ethylene concentration from 23 September onwards. The results corroborate the similar finding by reduction in values of ethylene with reduction in fan pressure.
Hydrogen/methane [H2/CH4] ratio
The hydrogen/methane ratio is an indicator of flaming combustion and increases with temperature. Since temperature could not be measured directly as electrical cables were laid in the borehole, the value of the index (T) was calculated from air sample analyses using Equation [10]
The results and fan pressures are presented in Figure 12.

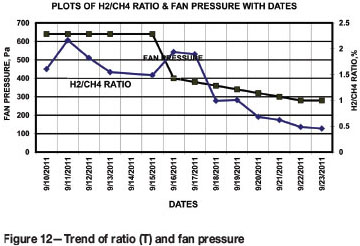
Figure 12 reveals that value of T, after reduction of fan pressure, increased to 1.9%, almost equal to the value on 10 September, and then decreased with reduction of fan pressure to a level of 0.45%. This may reflect the reduction in exchange of air after the implementation of control measures and a subsequent increase in the endothermic reaction in the fire-affected area. The trend also indicates a reduction in temperature at the seat of the fire.
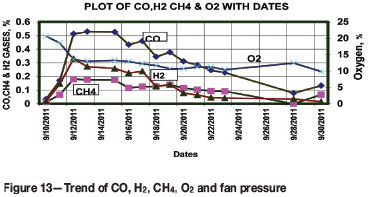
Trending of CO, H2, CH4, and O2
The trends of CO, H2, CH4, and O2 are presented in Figure 13. The results show a decrease in the values of CO, H2, CH4, and O2 from 0.5259%, 0.1762%, 0.259%, and 13.3% to 0.0786%, zero, 0.0352, and 12.43% respectively. Hence the environment of the fire may be considered as being oxygen-rich.
From the results of analyses of air samples and the assessment of the status of the fire, it was concluded that the intensity of fire had reduced to a smouldering/superficial state but combustion was continuing. In this situation, sealing of the fire area was called for. On the basis of the results of air samples, the underground environment was assessed as being non-explosive as confirmed by the Explo software based on Ellicott's extension of Coward's diagram (Vutukuri & Lama, 1986) for prediction of the explosibility of mine air mixture.
Finally, personnel under rescue cover were deployed underground on 10 October 2011 to isolate the fire area by erecting stopping at 1DE and 2DE between 27 L and 28L. The task was completed the same day. Subsequently, production in the mine was resumed on 12 October 2011.
Conclusions
A modified ventilation control technique was applied to deal with an open underground fire located in a critical situation in Surakachhar 3 & 4 Inclines mine, (SECL), Bilaspur, India. Sealing upstream of the fire, short-circuiting of air flow rate at the nearest location to the seat of the fire, and reduction in the effect of fan pressure and barometric pressure over the fire, based on results from monitoring the status of the fire, were effective in controlling the fire. This methodology is general enough to apply elsewhere under similar situations.
Acknowledgements
The authors are thankful to the CSIR-Central institute of Mining & Fuel Research institute (CIMFR), Dhanbad, for permission to publish this paper. The authors also acknowledge the members of Mine ventilation Discipline, particularly Sri A. Ansari, Technician, and Sri R.P. Dasaundhi, Laboratory Assistant, for support in field investigation. We thank the mine management, Sri S.N. Mishra (Manager), Sri Ajoy Kumar (Survey Officer), and Sri Z.H. Khan (General Manger) for their cooperation and support in during the investigation. The guidance and suggestions of Sri B.C. Bhowmick, Ex. Scientist and r Head, Mine Ventilation Discipline, CSIR-CIMFR, Dhanbad, is gratefully acknowledged. The opinions expressed in the paper are those of the authors and not necessarily those of CSIR-CIMFR, Dhanbad and M/s South Eastern Coalfield Limited, Bilaspur, India.
References
Banerjee, S.C. 2000. Early detection of heating and assessment of status of fire in mines, Prevention and Combating Mine Fires. Oxford & IBH, New Delhi, India Chapter-6. p. 165. [ Links ]
CIMFR. 2004. Studies on simulation of open fire in mine fire model gallery under varied airflow rate for suppression of fire and explosion in coalmines. Central Institute of Mining and Fuel Research, Dhanbad, Jharkhand, India. [ Links ]
Gillies, S., Wu, H.W., and Humphreys, D. 2004. Case studies from simulating mine fires coal mine and their effect on mine ventilation system. Australasian Institute of Mining and Metallurgy Branch, Coal Operators Conference, University of Wollongong, New South Wales. pp. 111-125. [ Links ]
Kissell, F.N. and Timko, R.J. 1991. Pressurization of intake escape ways with parachute stoppings to reduce infiltration of smoke. Proceedings of the 5th US Mine Ventilation Symposium, West Virginia. pp. 28-34. [ Links ]
Litton, C.D., Derosa, M.I., and Lí, J.S. 1987 Calculating fire-throttling of mine ventilation airflow. Report of Investigation RI 9076. US Bureau of Mines. [ Links ]
Liu, L and Kim, A.K. 2000. A review of water mist fire suppression systems: fundamental studies. Journal of Fire Protection Engineering, vol. 10, no. 3. pp. 32-50. [ Links ]
McPherson, M.J. 1993a. Subsurface Ventilation Engineering. Chapman and Hall, London. [ Links ]
McPherson, M.J. 1993b. Development and control of open fires in coal mine entries. Proceeding of the 6th US Mine Ventilation Symposium, University of Utah, Salt Lake City, 21-23 June. pp. 197-202. [ Links ]
Pandey, B.P., Choudhary, P., Singh, A.K., and Mendhe, V.A. 2003. Laboratory study of channel gasification with steam-air blast in sub-bituminous coal from Pindra Raniganj coalfield. Minetech, vol. 24, no. 6. pp. 37-49. [ Links ]
Ray, S.K, Singh, R.P., Sahay, N., and Varma, N.K. 2004. Assessing the sealed fire in underground coal mines. Journal of Scientific and Industrial Research, vol. 63. pp. 579-591. [ Links ]
Sahay, N., Bhowmick, B.C., Varma, N.K., Ray, S.K., Verma, S.M., and Mondal, P.K. 2001. Control of fire in a longwall panel under shallow cover with chamber method of ventilation and high pressure high stability nitrogen foam - a case study. Proceedings of the 7th International Mine Ventilation Congress, Krakow, Poland. pp. 971-978. [ Links ]
Singh, R.P., Ray, S.K,, Sahay, N., and Bhowmick, B.C. 2004. Study on application of fire suppression techniques under dynamic fire condition. Journal of the South African Institute of Mining and Metallurgy, vol. 104, no. 11. pp. 607-616. [ Links ]
Sahay, N., Sinha, A., Chakravorty, R.B., Prasad, P., Ahmad I., and Varma, N.K. 2008. Control of open fire in underground at Noonidih-Jitpur Colliery, SAIL [Steel Plant]. Journal of Mines Metals and Fuels, vol. 56, no. 12. pp. 241-248 [ Links ]
Urosek, J.E., Beiter, D.A., Stoltz, T.T., and Francart, W.J. Not dated. Recent United State coal mine fires lessons learned. http://www.msha.gov/ s&hinfo/techrpt/MEO/Recent United States Coal Mine Fires Lessons Learned.pdf. [ Links ]
Voracek, V. 1994. Use of nitrogen foam for both prevention and suppression of spontaneous combustion of coal in Ostrava Karvina Coalfields. Proceeding of the Workshop on Occupational Safety and Environment Protection in Underground Coal Mining Industry, SCZYRK, Poland. [ Links ]
Vutukuri, V.S. and Lama, R.D. 1986. Explosibility of mine atmospheres and fire gases, Environmental Engineering in mines, Cambridge University press, Melbourne 3166, Australia [ Links ]
Xie, J., Xue, S., Cheng, W., and Wan, G. 2011. Early detection of spontaneous combustion with development of an ethylene enriching system. International Journal of Coal Geology, vol. 85, no. 1. pp. 123-127. [ Links ]














