Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.114 n.2 Johannesburg Feb. 2014
PRECIOUS METALS CONFERENCE 2013
Synthesis and crystal structure of tetrakis(1,3-diphenyl-1,3-propanedionato) zirconium(IV)
M. Steyn; H.G. Visser; A. Roodt
Department of Chemical, University of the Free State, Bloemfontein, South Africa.
ABSTRACT
The coordination compound teirakis(1,3-diphenyl-1,3-propanediona to)zirconium(IV), [Zr(DBM)4], (DBM = dibenzoyl methane/1,3-diphenyl-1,3-propanedionol) was synthesized and characterized by single-crystal X-ray diffraction. [Zr(DBM)4] crystallizes in the monoclinic space group P21/c (a = 24.769(4) Å, b = 10.216(5) Å, c = 19.314(4) Å, and β= 101.541(5)°). The coordination geometry around the zirconium atom, formed by the eight O-donating atoms of the four β-diketonates, is found to be a near-perfect square antiprism. Crystal packing is stabilized by C-H...π interactions throughout the crystal lattice. This structure is a noteworthy example of the application of ambient condition synthesis of zirconium compounds in N,N'-dimethylformamide (DMF) as reaction and crystallization solvent, highlighting the possibilities of aerobic condition metal purification through chelation.
Keywords: zirconium, β-diketone, square-antiprismatic coordination polyhedron.
Introduction
Zirconium and its organometallic complexes have featured in a wide range of research studies for several years (Hoard and Silverton, 1963; Von Dreele, Stezowski, and Fay, 1971; Clegg, 1987; Calderazzo et al., 1998). Zirconium, with its very low affinity for thermal neutrons (radioactive energy), high thermal stability, and exceptional anti-corrosive properties, is widely used as cladding material for nuclear reactor fuel rods (Weast, 1982). Purification of zirconium from its minerals is known to be a laborious task, broadly utilizing dangerous acids and highly hazardous thermal conditions (Lowe and Parry, 1976; Nielsen, Schlewitz, and Nielsen, 2000; Speight, 2010).
The purification method that appears to be a significant point of interest in certain literature fields (Smolik, Jakobik-Kolon, and Poranski, 2009; Taghizadeh et al., 2009; Taghizadeh, Ghanadi, and Zolfonoun, 2011) is ion exchange purification - a method that involves the filtering of specific metal oxides through acidification processes along ion-exchange columns (Benedict, Schumb, and Coryell, 1954; Machlan and Hague, 1962; Qureshi and Husain, 1971). The main principles of organometallic compound behaviour in both solid state and solution are of substantial interest when considering the design of newer variations of such ion exchange purification methods.
As part of an ongoing study investigating coordination behaviour of O,O'- and N,O-bidentate ligands with zirconium(IV) and hafnium(IV) for possible influencing factors in the purification of these metals from base ore sources, we have been able to refine methods of synthesis and crystallization of zirconium complexes. This is in particular as a reference and a comparison with much older methods and results reported or published in the literature. In this paper we describe and elaborate on the synthesis and crystallographic characterization of the structure [tetrakis(1,3-diphenyl-1,3-propanedionato)- zirconium(IV)] as a redetermination and comparison to a previously published structure (Chun, Steffen, and Fay, 1979), and as a comparison to the hafnium(IV) counterpart (Viljoen, Visser, and Roodt, 2010).
The intimate geometric properties in the immediate coordination sphere of the Zr(IV) metallic molecule are essentially reproduced here, but with correspondingly elaborative statistical implication, indicating that the susceptibility of chelation geometry to intermolecular forces is greater than that of ligand internal geometry, as also previously published for the standard tetrakis(acetylace-tonato)zirconium(IV) ([Zr(acac)4]) structure (Clegg, 1987).
A schematic of Zr(DBM)4 is shown in Figure 1.
Experimental
Materials and instruments
All the starting chemicals and solvents were of analytical grade, commercially purchased and used without further purification. Synthesis was conducted under ambient laboratory conditions. All 1H NMR spectra were obtained in acetone-d6 on a Bruker 300 MHz nuclear magnetic resonance spectrometer.
Synthesis of [Zr(DBM)4]
ZrCl4 (101.2 mg, 0.434 mmol) and dibenzoylmethane (DBM) (396.6 mg, 1.769 mmol) were separately dissolved in DMF (10 ml each) and heated to 60°C. The DBM solution was added dropwise to the zirconium solution and stirred at 60°C for 30 minutes. The reaction solution was removed from heating, covered and left to stand for crystallization. Colourless trapezoidal crystals, suitable for single-crystal X-ray diffraction, formed after 21 days. (Yield: 382 mg, 88%). 1H NMR (300 MHz, acetone-d6): δ = 9.04 (dd, 1H), 8.13 (dd, 1H), 7.72 (q, 1H), 7.62 (s, 1H).
X-ray crystal structure determination
The X-ray intensity data was collected on a Bruker X8 ApexII 4K Kappa CCD area detector diffractometer, equipped with a graphite monochromator and MoKa fine-focus sealed tube (λ = 0.71069 Å, T = 100(2) K) operated at 2.0 kW (50 kV, 40 mA). The initial unit cell determinations and data collections were done by the SMART software package (Bruker, 1998a). The collected frames were integrated using a narrow-frame integration algorithm and reduced with the Bruker SAINT-Plus and XPREP software packages (Bruker, 1999) respectively. Analysis of the data showed no significant decay during the data collection. Data was corrected for absorption effects using the multi-scan technique SADABS (Bruker, 1998b), and the structure was solved by the direct methods package SIR97 (Altomare et al., 1999) and refined using the WinGX software (Farrugia, 1999) incorporating SHELXL (Sheldrick, 1997). The final anisotropic full-matrix least-squares refinement was done on F2. The aromatic protons were placed in geometrically idealized positions (C-H = 0.93 -0.98 A) and constrained to ride on their parent atoms with Uiso(H) = 1.2Ueq(C). Non-hydrogen atoms were refined with anisotropic displacement parameters. The graphics were obtained with the DIAMOND program (Brandenburg and Putz, 2006) with 50% probability ellipsoids for all non-hydrogen atoms.
Results and discussion
Most literature reports on the synthesis of zirconium(IV) compounds with β-diketones, and most reports on work in which where N- and O-donating bidentate ligands were used emphasize the importance of working under anaerobic conditions employing Schlenk-type apparatus. This is of course not viable in industrial applications like the purification of metal ores by organometallic reactions. The synthesis of [Zr(DBM)4] in DMF under aerobic conditions opens up new possibilities in the rich coordination chemistry of zirconium complexes.
The title compound, [Zr(DBM) 4], crystallizes in the monoclinic space group, P21/c. The asymmetric unit consists of a Zr(IV) metal ion coordinated to four unique, bidentate, oxygen-donating, β-diketonato ligands. The molecular structure of the title compound is represented in Figure 2 together with the atom numbering scheme. General crystallo-graphic details are presented in Table I, while selected bond lengths, bond angles, and torsion angles are listed in Table II.
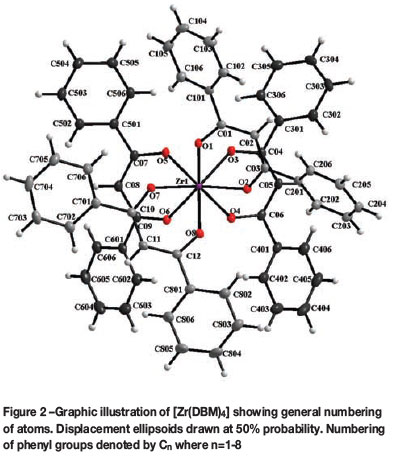
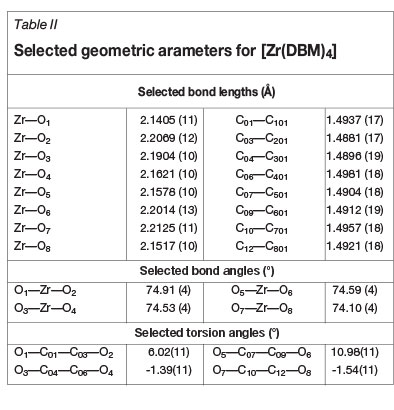
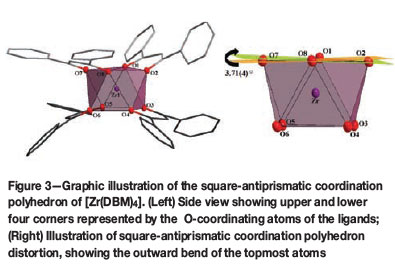
In this structure the Zr-O bond distances range from 2.141(1) Å to 2.2125(1) Å while the average O-Zr-O bite angle is 74.53(4)° (Table II). The four DBM-ligands are arranged around the metal centre in a space-filling, fan-like arrangement to give a square-antiprismatic coordination polyhedron (Figure 3a), with an almost negligible outward distortion towards dodecahedral geometry. This distortion of the ideal square antiprism lies with an outward bend of 3.71(4)°, as illustrated in Figure 3b.
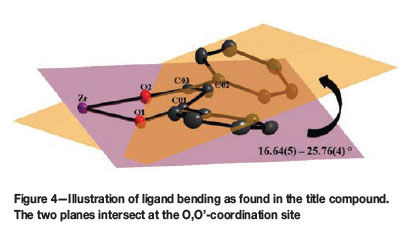
A noteworthy characteristic of the zirconium metal centre is observed in this structure, in that it distorts the backbone of the bidentate (acac-type) ligands from its preferred coordination geometry of approx. 180° towards a highly distorted bent-like geometry to accommodate the overall polyhedron geometry around the metal centre. These ligands are chelated around the metal centre with a bend in the ligand at the intersection of the two planes formed by the ligand-backbone (O-C-C-C-O) and the O-Zr-O bite angle (Figure 4). The extent of this ligand distortion ranges from 16.64(5) to 25.76(4)°.
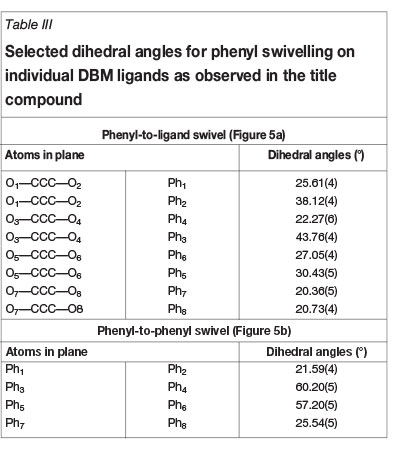
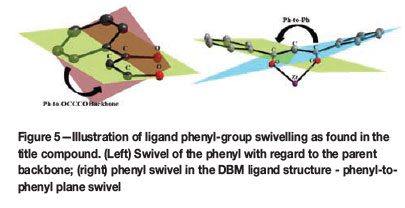
The ligand backbone itself also displays a twisting/bending in the arrangement of each individual phenyl ring at the edges of the C-C-C-backbone. Each individual Ph ring is swivelled at a distinct angle (see Table III) with respect to its parent β-diketone structural plane (Figure 5a), ranging from 20.36(5) to 43.76(4)°. Furthermore, the angle at which each phenyl pair are swivelled away from each other over the β-diketone structural plane ranges from 21.59(4) to 60.20(5)° (Figure 5b). This planar swivelling found for each individual phenyl ring is directly related to the manner in which the crystal lattice packing is arranged, as described below.
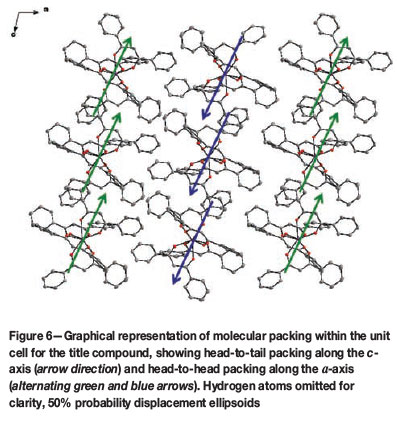
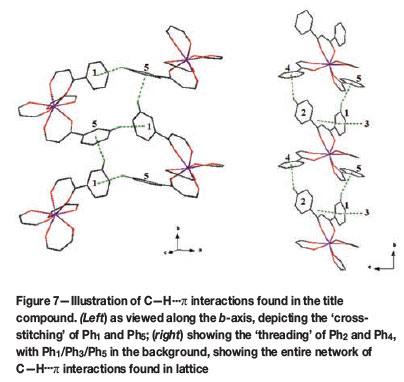
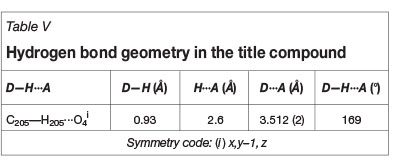
The compound itself packs in two observable ways in the lattice as a whole. Firstly, every organometallic molecule packs on top of another in a head-to-tail fashion along the c-axis, and head-to-head along the α-axis, as illustrated in Figure 6. Secondly, the most significant packing effect observed is a C-H...π interaction network, rigidly threading the entire crystal lattice together as a whole. This elaborate C-H...π interaction system found in the title compound is the cause for the unique swivelling of each individual phenyl ring on each DBM ligand.
The effect of this best described, as illustrated in Figure 7, as a 'cross-stitching' and 'threading' effect in the channelling of the head-to-tail packing along the c-axis in the crystal lattice. On one side of the stacked molecules, with a rigid 'cross-stitching' of the C-H...π interactions of Ph1, Ph3, and Ph5 (listed in Table IV), an intricate lattice assemblage of the symmetrically identical groups interacting and packing on their respective neighbours is observed. On the other side of the head-to-tail packed molecules, Ph2 and Ph4 show a looser, but still significant 'threading' in their symmetrically placed neighbours (Figure 7b). This intricate network of C-H...π interactions gives rise to the fact that no solvent molecules are caught inside the crystal lattice.
Although there are no classical hydrogen bonding or π-π stacking interactions, there is a weak intermolecular C-H... O interaction between C205-H205 of the Ph2 and an oxygen atom, O4, on a neighbouring molecule (listed in Table V).
Furthermore, this very stable arrangement of the organometallic molecules as a whole leads to two notable observations with regards to the nature of solid-state behaviour of zirconium and its β-diketone chelated compounds. Firstly, it is a well-known fact that zirconium β -diketone complexes in general always show a square-antiprismatic coordination polyhedron (Hoard and Silverton, 1963; Clegg, 1987). This suggests that zirconium has a certain preference for the chelation sites of the coordinating atoms, regardless of the steric properties of the ligands as a whole (Steyn, Roodt, and Steyl, 2008; Steyn et al., 2011; Steyn, Visser, and Roodt, 2012, 2013). However, the packing of each individual zirconium bidentate-ligand complex seems to be governed largely by the stabilization of the ligands themselves, and not from any discernable effect from the metal centre. This, in theory, could be due mainly to the fact that zirconium(IV) tends towards a maximum state of coordination, as preference, or as lowest crystallization state. This is in accordance with what is expected in these symbiotic systems (Huheey, Keiter, and Keiter, 1993).
In other words, since the metal centre is entirely surrounded by the specific bidentate ligands coordinated, it has no influence on the packing of the organometallic molecule in the greater crystal lattice, but only influences the placement of the coordinating atoms around itself, thereby not allowing for dimeric structures or other metal-to-linking-atom interactions.
As far as the physical structural characteristics of this tetrakis(β-diketone) zirconium(IV) complex are concerned, all aspects are in good comparison to other published structures containing β-diketone ligands (Table VI). All coordination bond lengths are in the average range of 2.1-2.2 A and bite angles average the standard angle of 74-75°. Furthermore, it is interesting to note that all these fully coordinated zirconium(IV) β-diketonates appear to prefer a monoclinic space group across the board. Finally, in comparison to the previously published structure (Chun, Steffen, and Fay, 1979), although the asymmetric unit appears identical and all structural characteristics are comparable (Table V), these two crystals are not completely identical. When comparing the smaller crystallographic cell volume for the title compound to that reported by Chun, Steffen, and Fay, there does appear to be a tighter packing of the crystal lattice here.
Regardless of the final crystallographic findings, however, it is significant to note that Chun, Steffen, and Fay reported a synthetic procedure of phosphite-catalysed reflux in diethyl ether and crystallization method of benzene/hexane extraction after vigorous purification of the reaction solution, for producing these specific crystals. This methodology in itself was most likely employed merely as a laboratory standard, but we can now report, with a certain amount of confidence, that zirconium as a metal reagent is more reactive than may have been expected in the past 30 years.
These rigorous synthesis methods and laborious crystallization techniques are not always necessary. Organometallic chelation reactions of zirconium are often self-catalysed and crystallization can occur in ambient environments. Furthermore, application of DMF as a general solvent in all processes allows for less strict approaches to the impacts of hydration on the final product, since crystalline water could also be observed in some cases in the asymmetric unit, without any influence on the main metal-molecule as a whole (Steyn, Roodt, and Steyl, 2008).
Conclusions
The improved synthesis of Zr(IV) complexes with β-diketone ligands has been illustrated here with tetrakis(1,3-diphenyl-1,3-propanedionato)zirconium(IV). It is shown that the intimate geometry around the metal atom seems to be governed largely by zirconium itself. A preference for square-antiprismatic coordination polyhedra for many O,O'- and N,O-bidentate ligand complexes across the board seem to indicate this fact (Chun, Steffen, and Fay, 1979; Viljoen, Visser, and Roodt, 2010; Steyn, Roodt, and Steyl, 2008; Steyn et al., 2011; Steyn, Visser, and Roodt, 2012, 2013). The ligand geometries do, however, play a vital role in the stability of the crystal lattice as a whole. In the case of the title compound, which yielded very stable crystals in ambient conditions, the C-H...π interactions of most of the coordinated DBM ligands thread the entire crystal lattice into a very stable three-dimensional network.
Acknowledgement
Financial assistance from the Advanced Metals Initiative (AMI) of the Department of Science and Technology (DST) of South Africa, through the New Metals Development Network (NMDN) coordinated by the South African Nuclear Energy Corporation Limited (Necsa) is gratefully acknowledged.
We also express our gratitude towards SASOL, PETLabs Pharmaceuticals, and the University of the Free State Strategic Academic Initiative (Advanced Biomolecular Systems) for financial support of this research initiative outputs. This work is based on research supported in part by the National Research Foundation of South Africa (SA-NRF/THRIP; UIDs 71836 & 84913).
References
Altomare, A., Burla, M.C., Camalli, M., Cascarano, G.L., Giacovazzo, C., Guagliardi, A., Moliterni, A.G.G., Polidori, G., and Spagna, R. 1999. SIR97: a new tool for crystal structure determination and refinement. Journal of Applied Crystallography, vol. 32. pp. 115-119. [ Links ]
Benedict, J.T., Schumb, W.C., and Coryell, C.D. 1954. Distribution of zirconium and hafnium between cation-exchange resin and acid solutions. The column separation with nitric acid-citric acid mixture. Journal of the American Chemical Society, vol. 76, no. 8. pp. 2036-2040. [ Links ]
Brandenburg, K. and Putz, H. 2006. DIAMOND, Release 3.0e, Crystal Impact GbR, Bonn, Germany. [ Links ]
Bruker AXS Inc. 1998a. Bruker SMART-NT Version 5.050. Area-Detector Software Package. Madison, WI. [ Links ]
Bruker AXS Inc. 1998b. Bruker SADABS Version 2004/1. Area Detector Absorption Correction Software. Madison, WI. [ Links ]
Bruker AXS Inc. 1999. Bruker SAINT-Plus Version 6.02 (including XPREP), Area-Detector Integration Software. Madison, WI. [ Links ]
Calderazzo, F., Englert, U., Maichle-Mossmer, C., Marchetti, F., Pampaloni, G., Petroni, D., Pinzino, C., StrAhle, J., and Tripepi, G. 1998. Eight-coordinate chelate complexes of zirconium(IV) and niobium(IV): X-ray diffractometric and EPR investigations. Inorganica Chimica Acta, vol. 270, no. 1-2. pp. 177-188. [ Links ]
Chun, H.K., Steffen, W.L., and Fay, R.C. 1979. Effects of crystal packing on the coordination geometry of eightcoordinate metal chelates. Crystal and molecular structure of tetrakis(1,3-diphenyl-1,3-propanedionato) zirconium(IV). Inorganic Chemistry, vol. 18. pp. 2458-2465. [ Links ]
Clegg, W. 1987. Redetermination of the structure of tetrakis(acetylacetonato) zirconium(IV). Acta Crystallographica C, vol. 43 pp. 789-791. [ Links ]
Farrugia, L.J. 1999. WinGX suite for small-molecule single-crystal crystallography. Journal of Applied Crystallography, vol. 32. pp. 837-838. [ Links ]
Hoard, J.L. and Silverton, J.V. 1963. Stereochemistry of discrete eight-coördination. II. The crystal and molecular structure of zirconium(IV) acetylacetonate. Inorganic Chemistry, vol. 2. pp. 243-249. [ Links ]
Huheey, J.E., Keiter, E.A., and Keiter, R.L. 1993.Inorganic Chemistry -Principles of Structure and Reactivity. 4th edn. HarperCollins College Publishers, New York. [ Links ]
Lowe, A.L. and Parry, G.W. (eds.). 1976. Zirconium in the Nuclear Industry: Proceedings of the Third International Conference, Quebec City, Canada, 10-12 August 1976. p.7. ASTM, Philadelphia, PA. [ Links ]
Machlan, L.A. and Hague, J.L. 1962. Separation of hafnium from zirconium and their determination: separation by anion-exchange. Journal of Research of the National Bureau of Standards, vol. 66A, no. 6. pp. 517-520. [ Links ]
Nielsen, R.H., Schlewitz. J.H., and Nielsen, H. 2000. Zirconium and zirconium compounds. Kirk-Othmer Encyclopedia of Chemical Technology, vol. 26. John Wiley & Sons. pp. 630-631. [ Links ]
Qureshi, M. and Husain, K. 1971.Quantitative cation exchange separation of zirconium and hafnium in formic acid media. Analytical Chemistry, vol. 43. pp. 447-449. [ Links ]
Sheldrick, G.M. 1997. SHELXL97. Program for crystal structure refinement. university of Gottingen, Germany. [ Links ]
Smolik, M., Jakobik-Kolon, A., and Poranski, M. 2009.Separation of zirconium and hafnium using Diphonix(R) chelating ion-exchange resin. Hydrometallurgy, vol. 95, no. 3-4. pp. 350--53. [ Links ]
Speight, J.G. 2010. The Refinery of the Future. William Andrew, London. pp. 134-136. [ Links ]
Steyn, M., Roodt, A., and Steyl, G. 2008. Tetrakis(1,1,1-trifluoroacetylace-tonato-k2O,O')zirconium(IV) toluene solvate. Acta Crystallographica, vol. E64. p. m827. [ Links ]
Steyn, M., Visser, H.G., and Roodt, A. 2012. Tetrakis(5,7-dimethylquinolin-8-olato- 2N,O)zirconium(IV) dimethylformamide disolvate. Acta Crystallographica, vol. E68. pp.m1344-m1345. [ Links ]
Steyn, M., Visser, H.G., and Roodt, A. 2013. Evaluation of oxine-type ligand coordination to zirconium (IV). Journal of the Southern African Institute of Mining and Metallurgy, vol. 113, no. 2. pp. 105-108. [ Links ]
Steyn, M., Visser, H.G., Roodt, A. and Muller, T.J. 2011. Tetrakis(picolinato-2N,O)zirconium(IV) dehydrate. Acta Crystallographica, vol. E67. pp. m1240-m1241. [ Links ]
Taghizadeh, M., Ghanadi, M., and Zolfonoun, E. 2011. Separation of zirconium and hafnium by solvent extraction using mixture of TBP and Cyanex 923. Journal of Nuclear Materials, vol. 412. pp. 334-337. [ Links ]
Taghizadeh, M., Ghasemzadeh, R., Ashrafizadeh, S.N., and Ghannadi, M. 2009. Stoichiometric relation for extraction of zirconium and hafnium from acidic nitrate solutions with Cyanex272. Hydrometallurgy, vol. 96. pp. 77-80. [ Links ]
Viljoen, J.A., Visser, H.G., and Roodt, A. 2010. Tetrakis(1,3-diphenylpropane-1,3-dionato)hafnium(IV). Acta Crystallographica, vol. E66. pp. m1053-m1054. [ Links ]
Von Dreele, R., Stezowski, J.J., and Fay, R.C. 1971. Crystal and molecular structure of chlorotris(acetylacetonato)zirconium(IV). Journal of the American Chemical Society, vol. 93, no. 12. pp. 2887-2892. [ Links ]
Weast, R.C. 1982. CRC Handbook of Chemistry and Physics. 63rd edn. CRC Press, Boca Raton, FL. [ Links ]
Zherikova, K.V., Morozova, N.B., Kurat'eva, N.V., Baidina, I.A., Stabnikov, P.A., and Igumenov, I.K. 2005. Journal of Structural Chemistry, vol. 46. pp. 513-522. [ Links ] ♦
†Electronic supplementary information (ESI) and complete crystallo-graphic detail in cif format are available on request.














