Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.114 n.2 Johannesburg Feb. 2014
PRECIOUS METALS CONFERENCE 2013
Ion exchange technology for the efficient recovery of precious metals from waste and low-grade streams
V. Yahorava; M. Kotze
Mintek, Randburg, South Africa
ABSTRACT
Efficient recovery of precious metals from process solutions is essential for improving process economics. Traditionally, precious metals are relatively effectively recovered from waste streams via precipitation or cementation. However, these approaches have a number of drawbacks, including poor water balance, creation of environmentally unfriendly waste streams, and losses of precious metals. Ion exchange technology is an alternative for the recovery of precious metals from waste or low-grade streams. This technology allows the recovery of the precious metals to extremely low levels (micrograms per litre) with relatively high upgrade ratios from the solution onto the resin without major water balance concerns, while the impact on the environment could be minimized or avoided.
Research was conducted on the recovery of platinum group metals and gold from different low-grade and waste streams from one of the precious metals refineries in South Africa by means of ion exchange. Various functionalities and matrices (granular and fibrous) of ion exchange materials were evaluated. The results from these studies indicated that in some cases ion exchange could be very effective for the recovery of precious metals, and that the PGM concentration could be reduced to < 1 mg/L. The upgrading ratios of the various PGMs onto the specific fibres were relatively high for the specific streams evaluated, which might in some cases justify incineration of the loaded material instead of stripping and recycling the adsorbent. The cost of direct incineration for one of the waste streams tested would be less than 1% of the value of the PGMs recovered. However, the adsorbent has to be carefully selected and the process design optimized for each specific stream.
Keywords: PGMs and Au refining, precious metals, waste streams, low-grade streams, ion exchange, resin, fibre, recovery.
Introduction
Waste streams generated during the refining of precious metals may contain significant amounts of platinum group metals (PGMs) and gold (Au). Currently, refineries are using mainly precipitation and cementation techniques for the recovery of valuable metals from low-grade and waste streams. However, there are major drawbacks in using these technologies, including incomplete recovery of valuable metals and generation of waste streams that carry significantly more contaminants than the original waste streams. There is therefore a need to use alternative technology to precipitation/cementation, which would allow the cost-effective and efficient recovery of precious metals, with low or minimal additional environmental implications.
Ion exchange and/or adsorption technology can resolve difficulties associated with the recovery of precious metals from low-grade and waste streams by minimizing waste generation and improving the overall economics of the process. Smopex® and SuperLig® materials are widely known as an option for recovery of valuable metals in the precious metals industry (Danks, n.d.; Izatt, Bruening, and Izatt, 2012). However, the cost of these materials (typically much greater than US$200 per kilogram) often prevents refineries from considering them for waste treatment.
Over the past few years extensive research has been performed on the application of conventional ion exchange (IX) materials for the recovery of PGMs and Au from various low grade streams. A number of South African refineries provided some of their low-grade streams, which are currently treated via precipitation or cementation for PGM and Au recovery. The current paper reports on a few successful examples of the potential of ion exchange technology for the treatment of such streams. Ion exchange can be designed to result in highly efficient recovery of PGMs and Au, often without the addition of further contaminants.
The test work programme for the evaluation of the various ion exchange materials for the recovery of precious metals from various streams was as follows:
►Selection of the most promising functional groups commercially available on ion exchange materias;
►Evaluation of granular and fibrous ion exchangers
►Comparison of equilibria and fixed bed breakthrough profiles.
Detailed results and information on the streams and materials that where tested are not provided in this paper due to confidentiality. Nevertheless, the major benefits of conventional ion exchange for the treatment of some low-grade/waste streams containing precious metals are highlighted.
Background
The precious metal refineries combine different methods to recover silver, gold, and PGMs (platinum, palladium, rhodium, iridium and ruthenium). Depending on the process used in a specific refinery, waste streams containing PGMs and/or gold have different chemical compositions, pH, and Eh values. Due to the competitive nature of the PGM refining business and the high value of the products, very little detail of the processes used by the individual South African refiners has been published. The refineries from which waste streams were sourced for this study restricted the publication of the detailed compositions of their waste streams in this paper.
The efficiency of IX for the recovery of various metals from low-grade refinery streams depends on the chemical characteristics of the stream. For example, PGMs form a number of complexes in chloride medium depending on Eh, pH/free acid concentrations, and chloride concentrations. The major PGM species in chloride medium are shown in Table I.
In chloride acid media PGMs are present mainly as anionic species and conventional anion exchange materials could be considered for their bulk recovery from low-grade streams.
IX, using commercially available adsorbents, might be a very cost-effective technology for the recovery of PGMs and Au from low-grade or waste streams, without the generation of more contaminated waste as is the case with precipitation and cementation.
IX materials traditionally used in hydrometallurgy are supplied in the form of granular resins. Recently, production of fibrous ion exchange materials was scaled-up, thus opening an opportunity for their evaluation and comparison with granular materials (Yahorava, Kotze, and Auerswald, 2013). Enlarged images of resin beads and fibre filaments are shown in Figure 1.
Granular IX materials, in fixed bed systems, have some disadvantages []:
►Slow reaction rates and diffusion: relatively large resin bead sizes (400-600 µm) are generally used in order to prevent excessive pressure drop in fixed bed columns, which results in relatively slow kinetics and diffusion. The main consequences of this are:
- Large ion exchange resin columns and resin inventories, so high CAPEX
- Higher OPEX due to kinetic constraints on achieving equilibrium, thereby limiting the operating capacity of the resin, hence increasing reagent consumption per mass of valuable metal
- Generation of relatively large, mixed volumes of solutions due to mixing occurring during diffusion when different process streams are passed through the resin bed (adsorption stream, wash streams, elution stream).
►Poor water balance:
- Generation of excessively large effluent streams (especially at low upgrading ratios onto the resin).
►Resin loss:
- Osmotic shock and excessive pressure results in resin loss through breakage, which increases the pressure drop across a fixed bed column and the OPEX (resin replacement). Also, resin breakage can result in a loss of valuable metal loaded onto the resin fines.
Fibrous ion exchange materials are filaments with diameters of 20-50 µm, containing ionizing and complexing functional groups (Soldatov, 2008). These materials can have the same functional groups to those on resins, and hence be applied for similar applications.
Fibres have the following advantages over granular analogues (Soldatov, 2008):
►Short diffusion paths that provide adsorption rates that can be up to hundred times faster than that of conventional granular resins (with particle diameters usually between 0.25 and 1 mm). This would result in a significant decrease in the CAPEX for the specific unit operation
►Higher osmotic stability and hence minimal losses, thereby decreasing the associated OPEX and valuable metal loss
►Pressure drop across a fibre bed is lower than in packed resin beds, as the compressibility of fibre is limited.
►Operating capacities are closer to theoretical capacities as a result of faster kinetics, hence reagent consumption and OPEX could be lower.
Both fibrous and granular ion exchange materials were evaluated for the recovery of PGMs and Au from low-grade or waste streams.
Experimental
Resin/fibre conversion
Portions of each of the selected exchangers were loaded into a column. Strong- and weak-base anion exchange resins/fibres were converted to the chloride form, while the chelating resin was converted to the H+-form by passing 4 bed volumes (BVs) of 1 M HCl through the column at a flow rate of 2 BV per hour. In order to remove entrained acid, the adsorbents were washed with deionized water until the pH of the effluent water reached approximately 2.
Screening tests
Portions of the converted (H+ or Cl- form) adsorbents were batch-contacted with the specific liquor at different volumetric or mass ratios for 24 hours at ambient temperature in rolling bottles. The contact ratio was calculated based on the capacity of the tested material and the estimated total concentration of precious metal complexes in the liquor.
After 24 hours, the solution was separated from the resin/fibre via screening through a 212 µm screen. The resins were washed with deionized water and oven-dried at 60°C. The solution samples and the resins were analysed for the metals of interest by inductively coupled plasma - optical emission spectroscopy (ICP-OES) or inductively coupled plasma - mass spectroscopy (ICP-MS) depending on the detection limit required. Detection limits of ICP-OES and ICP-MS are >5 mg/L >10 µg/L respectively.
Equilibrium loading/adsorption isotherm
The ion exchange materials to be used for the generation of the equilibrium loading isotherms were selected based on the preliminary screening tests. If promising materials were available in both fibrous and granular forms, both adsorbents were tested. The solution was batch-contacted with the chosen adsorbents at different resin-to-solution ratios (mass ratios for fibre and volumetric ratios for granular resins) for 24 hours in a similar manner as described above. After 24 hours, the adsorbents were separated from the solution and barren solution samples were collected. The adsorbents were then washed with de-ionized water and oven-dried at 60°C. Solution samples and dried adsorbents were analysed for the metals of interest.
Column breakthrough test work
Column breakthrough tests were conducted by passing the feed solution through a fixed bed of resin/fibre at a specified flow rate. The column was charged with wet resin, and then filled with water. The resin bed was stirred with a spatula to remove any air bubbles prior to actual solution being passed through the column. Dry fibre was packed into the column, which was wetted by passing water upwards through the column to limit air entrainment in the fibre bed and solution bypass during operation. Hence, during the test work liquors flowed downwards through the granular resin beds and upwards through the fibre beds.
The solution throughput was estimated based on the results of the equilibrium loading isotherm tests. Barren solution samples were collected at specific bed volume intervals. After the bed had reached saturation level (>80% of the metals targeted was detected in the effluent), it was washed with deionized water to remove entrained solution. The adsorbents were then oven-dried at 60°C. Solution and adsorbent samples were analysed for the metals of interest via ICP-OES or ICP-MS.
Fibres were tested in the form of staple; they were packed into the column to 0.33 g/cm3 packing density (absolutely dry fibre). This density was similar to the density of the resins tested (d50 of the resins included in the evaluation varied between 500 and 600 µm).
Since the height of the column was 20 cm and the diameter was 1.4 cm, the column was charged with approximately 30 mL of resin and 10 g of fibre.
Results and discussion
Brief description of low-grade PGM streams
Result of the evaluation of three low-grade PGMs streams are reported:
►'Pd Waste' - barren liquor after precipitation and filtration of (Pd (NH3)2Cl2
►'Pt Waste' - barren liquor after precipitation and filtration of NH4PtCl6
►'Mixed Waste' - a combination of various low-grade PGMs and Au streams prior (taken before neutralization and sulphidization).
During the test work programme a number of batches of solution were obtained, and the concentrations of the precious metals as well as Eh and pH values varied. Results generated for some of the streams (Pd and Pt wastes, Mixed Waste) are reported in this paper. However, the impact of the variation in feed composition has to be well understood in order to ensure optimum process design.
IX materials tested
The typical speciation of the precious metals (Table II) in the respective solutions was used in the selection of appropriate ion exchangers.
Abbreviations used for the ion exchange materials as well as some of characteristics and indicative prices for the granular resins are listed in Table II.
Strong acid cation exchanger was included in the evaluation as examples of its successful application for precious metals recovery have been reported in the open literature (Boehm and Hampel, 1972; Borg et al., 1955). IDA resin was tested as it exhibits the properties of an anion exchanger in solutions of pH < 2 or less as both carboxylic groups and nitrogen occur in the protonated form (Hubicki et al., 2006).
Adsorbents 1, 3, and 5 are commercially available in fibrous and granular form. Other adsorbents can be produced on a fibrous matrix on request if a market need is identified. Generally, the price of fibrous materials is approximately 25 % higher than that of granular ones (mass basis comparison).
Ion exchange fibres are usually supplied in the air dry form as yarn, staple, threads, non-woven materials, or webs and sold on the mass basis. Ion exchange resins typically are sold on the wet volume basis. In order to compare fibre and resin performance it was assumed that once packed into the column and pressed, fibre will have density of 0.33 g/cm3.
Screening of IX functional groups
Palladium and Platinum Waste streams
The compositions of the Pt Waste, Pd Waste, and Mixed Waste streams used for the screening tests are given in Table III. Base metal concentrations were less than than 5 mg/L in the streams tested.
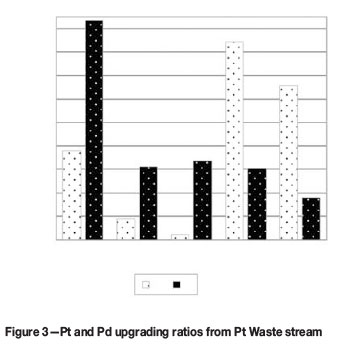
Adsorbents were contacted with portions of solution in a 1-to-10 (v/v) ratio. Results of screening various functional groups (Table I) for the recovery of Pt and Pd from the Pd and Pt Waste streams are presented in Figure 2 and Figure 3 respectively.

The adsorbents were evaluated with regard to Pt and Pd upgrading ratios only as the other impurities were present in concentrations close to or below the detection limit of ICP-OES (<5 mg/L). During selecting the most suitable material for treatment of the Mixed Waste solution, all the PGMs were traced.
Upgrades were calculated as follows:

where
q - metal concentration on resin, mg/L
Mebarren -metal concentration in solution after contact, mg/L.
The highest Pd and Pt upgrades from both streams were achieved by Adsorbents 1, 4, and 5 having anion exchange functionalities (see Table I). For the Pd Waste stream (Figure 1) Pd was upgraded more than a 100 times with Adsorbents 1, 4, and 5, while the upgrading of Pt onto these adsorbents was negligible. This was probably due to preferential adsorption of Pd chloride species of Pt at the conditions tested.
For the Pt Waste stream, the following results were found:
►Adsorbent 1: Pt and Pd upgrade ratios of >180 and > 60 respectively
►Adsorbents 4 and 5 showed higher upgrades for Pd (>120) than for Pt (<60) ;
Adsorbent 4 was excluded from further test work as no fibrous analogue was available, but it could be considered in future.
Mixed Waste stream
Results of the adsorbent screening tests on the Mixed Waste solution are presented in Figure 4. Only Adsorbent 1 was found to be promising for Pt and potentially Rh recovery from this liquor.

Equilibrium adsorption isotherms
Palladium and Platinum Waste streams
Equilibrium adsorption isotherms for Pd and Pt recovery from the relevant waste streams were generated using Adsorbents 1 and 5 in fibrous and granular form. The compositions of the solutions used are given in Table IIIIII.
Figure 5 illustrates the equilibrium isotherms for Pd and Pt obtained with the Pd Waste stream. Resin/fibre loadings were calculated by using feed and barren solution analysis. In order to convert metal loadings onto fibre into mg/L, a fibre compression density of 0.33 g/cm3 was used. In some cases, a full equilibrium isotherm was not achieved due to an insufficient amount of solution available for the test work.
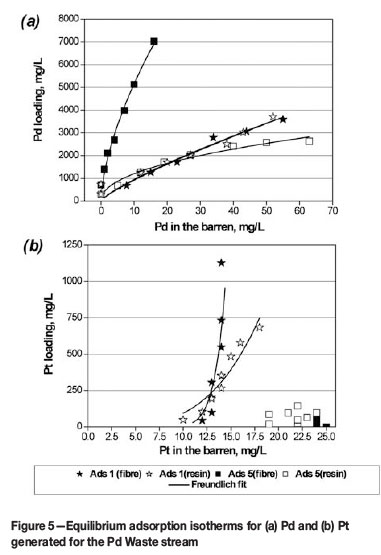
Adsorbent 1 (TETA functional groups), in fibrous and granular forms, showed similar equilibrium behaviour towards Pd and Pt:
►Comparable maximum Pd loadings of approximately 3.5 g/L were achieved, giving upgrade ratio of about 65-70
►Upgrading ratios for Pt at around 13 mg/L Pt in the equilibrium solution were 80 and 30 for the resin and fibre respectively.
The following results were obtained with Adsorbent 5 (TBA functional groups):
►The Pd loading onto the resin at >40 mg/L Pd in solution was around 2.8 g/L, which was a <50 times upgrade
►The fibrous IX material resulted in a Pd loading of 7 g/L at a Pd solution concentration of 18 mg/L in solution, which was a >380 times upgrade
►Very little Pt loading was obtained on either the granular or fibrous materials.
Based on the results from these scouting tests, it seemed that Adsorbent 1 would be successful for the recovery of Pt and Pd, but Adsorbent 5 might be useful in the selective recovery of Pd from streams containing Pt. The performance of this fibre in the actual separation of Pd from the other PGMs during refining should also be further evaluated.
Due to the unfavourable shape of the equilibrium isotherm and impossibility of reducing Pt concentration to <10 mg/L, this type of material was excluded from further evaluation for this specific stream. The reason for inefficient Pt extraction from the Pd waste stream might be due to the formation of poorly extracted Pt species under the conditions tested.
Equilibrium adsorption isotherms generated for Pt and Pd recovery from the Pt Waste stream are presented in Figure 6.

None of the tested materials reached their final maximum loadings for this specific liquor, as the barren concentrations were still considerably lower than that of the feed concentration at the maximum ratio tested (1:500 resin- to-liquor m/m).
Adsorbent 1 showed higher recovery efficiencies towards Pt, while Adsorbent 5 extracted more Pd. Maximum Pd and Pt upgrades achieved with Adsorbent 5 were around 860 and 100 respectively, while Adsorbent 1 produced upgrades of 240 and 680 for Pd and Pt respectively at the maximum solution-to-adsorbent ratio (v/v) of 500.
Adsorbent 5, containing strong base functional groups on both fibrous and granular forms, was chosen for further evaluation of Pd recovery from the relevant waste stream, while Adsorbent 1 with polyamine functionality, was tested for Pt recovery from the platinum waste stream.
New batches of waste streams were received, and the compositions are given in Table IV. Concentrations of other PGMs in the feed were close to the ICP-OES detection limit of 2 mg/L and were not monitored further.

Equilibrium isotherms generated for the recovery of Pd and Pt from the second batch of Pd and Pt Waste streams with fibrous and granular ion exchangers carrying TBA and TETA groups are presented in Figure 7 and Figure 8 respectively.
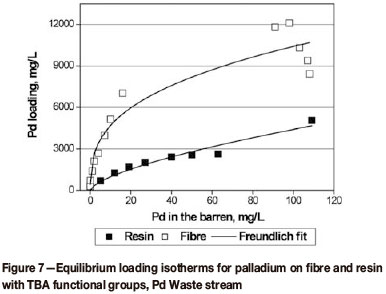

Maximum loadings of palladium achieved from the Pd Waste solution with strong-base fibre and resin (Adsorbent 5) were 10.5 g/L and 5 g/L, respectively, at equilibrium Pd barren concentrations of 110 mg/L. Upgrades of approximately 100 and 50 were achieved for the fibre and resin respectively; hence recovery of the Pd from the loaded adsorbents via incineration would probably not be economically feasible. Therefore, recovery of the metal from the adsorbent by elution should be considered. Eluants such as 6 M HCl, acidic thiourea, and ammonia bisulphite might be suitable (Korkisch, 2000; Bernardis, Grant, and Sherrington, 2005).
The maximum Pt loadings achieved with both fibrous and granular ion exchangers (Adsorbent 1) from the Pt Waste stream were similar at about 60-75 g/L resin/fibre, at an equilibrium barren Pt concentration of about 250 mg/L, hence upgrading ratios of >200 times. Based on the relatively high upgrade achieved, the current price of Pt metal (i.e. 45 818 US$ per kilogram on 13 March 2014) and the cost per kilogram of adsorbent (about US$50), it might be cost-effective to recover the metal by incineration of the adsorbent instead of elution.
Table V presents preliminary costs for platinum recovery from the low-grade waste stream via ion exchange technology (resin or fibre with weak base polyamine functionality can be used) followed by incineration of the loaded material.

The cost of the material, if incinerated and not recycled, would be less than 1% of the value of metal recovered. This might allow for incineration to be economically attractive. Ion exchange columns/cartridges could easily be introduced into the refinery for waste treatment without excessive capital costs, and tested on site.
Mixed Waste stream
Equilibrium adsorption isotherms for platinum recovery from the Mixed Waste stream (Table III) with Adsorbent 1 are presented in Figure 9. The upgrading ratio for Pt was about 140 for both adsorbents.
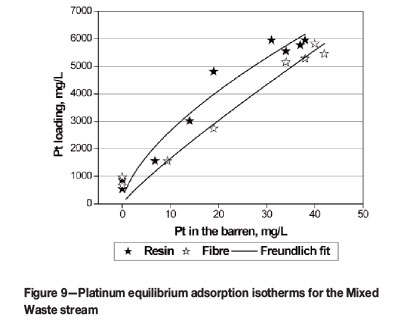
The Pt adsorption equilibrium of the resin was somewhat more favourable than that of the fibre. Based on these results and those obtained for the recovery of Pt from the Pt Waste stream, it was clear that the target would generally be to recover Pt and Pd from their individual waste streams and not from a mixture in order to obtain improved performance from the IX system.
There was very little Pd in the Mixed Waste stream, but the Ru concentration was relatively high. However, the loadings observed for these metals were <0.1 g/L.
Breakthrough tests with fibrous and granular IX materials
Pd Waste Stream: breakthrough
Breakthrough profiles for the recovery of Pd from the Pd waste stream (Table V) using granular and fibrous Pd-selective ion exchange materials (Adsorbent 5) are shown in Figure 10.

Pd breakthrough (>1 mg/L in barren) was reached after passing approximately 21 BVs of solution through the resin bed and approximately 93 BVs through the fibre column at a flow rate of approximately 2 BV/h. Various solution flow rates were evaluated for the fibre: namely, 2, 5, 10, and 15 BV/h. Results indicated that the Pd mass transfer zone time for the fibre was highly dependent on flow rate. A summary of the results achieved as well as some preliminary design parameters is given in Table VI.

Indicative adsorbent requirements for a plant treating 100 L/h of a waste stream containing approximately 100 mg/L Pd were generated assuming that:
►Solution will be passed through a lead-lag column arrangement
►Once the resin/fibre in the first column reaches approximately 80% palladium breakthrough, the column will be removed from the system and the loaded adsorbent incinerated for Pd recovery
►A column containing fresh adsorbent will be placed in the lag position.
Treatment of the Pd Waste stream using IX resulted in effective palladium recovery (>99%) leaving <1 mg/L in the barren solution. Some co-loading of Pt was observed (Table VI), which might be beneficial for the specific application.
The fibre IX system would have the following advantages over a granular system:
►Higher Pd upgrade (>2 times), hence lower adsorbent flow rate
►The fibre column could be operated at about 95% breakthrough, hence closer to theoretical equilibrium, than the resin column, which would be operated at about 80% breakthrough
► Faster adsorption kinetics and hence significantly smaller inventory requirements;
Use of fibrous instead of granular ion exchange material for the treatment of the Pd Waste stream allowed a decrease in the size of the plant by >70%. Also in this specific case, the flow rate of fibre was approximately half that of resin, and hence the incineration costs would be significantly reduced. Fibres are generally more expensive than granular resins (mass basis), hence a cost comparison would have to be done to identify the most cost-effective option for a specific application. However, efficient elution employing a suitable reagent (HCl, thiourea, etc.) should make the recovery of Pd from low-grade or waste streams using ion exchange technology highly attractive.
Mixed Waste solution: breakthrough
Results for the recovery of Pt from the Mixed Waste solution (Table IV) using Adsorbent 1, in the fibrous and granular forms, are presented in Figure 11. Results and some preliminary design parameters are listed in Table VII.


Both fibre and resin were effective for the recovery of Pt from the Mixed Waste stream. The loaded adsorbents contained primarily Pt and some Rh. The ratios of Pt to Rh in the feed liquor and on the loaded ion exchangers were similar, indicating that Adsorbent 1 was effective for the simultaneous recovery of Pt and Rh, as the selectivity coefficients for Pt over Rh were about 1.
Similar final Pt loadings of 8.4-8.9 g/L were achieved with the fibre and resin. However, for a full-scale plant treating about 100 L/h of waste containing approximately 50 mg/L Pt, the plant using fibre could be > 40% smaller than the equivalent granular ion exchange plant.
Recovery of all the PGMs from the Mixed Waste solution by using an exchanger with a TETA type of adsorbent was ineffective. Potentially, it is possible to improve recovery of the Pd, Ir, and Ru by introducing another type or types of ion exchange materials into the treatment sequence. Also, an efficient elution procedure must be developed for the recycling of the adsorbents to make the process economically viable for such types of streams.
Conclusions
Evaluation of ion exchange technology for the recovery of precious metals from low grade or waste streams indicated that:
►It was possible to decrease the PGM tenor in the effluent streams to <1 mg/L
►Commercially available ion exchange materials with triethyltetramine and tributylamine functional groups were found to be suitable for recovery of Pt, Rh, and Pd respectively with high upgrade ratios
►Pt-selective resins/fibres were capable of loading up to 17% platinum with the cost of direct incineration of materials loaded in some cases being less than 1% of the value of the metals recovered.
Optimization of adsorption parameters and further conditioning of the low-grade liquor prior to contact with ion exchange material might improve upgrades and maximum loadings of the PGMs.
Two types of ion exchange materials, fibrous and granular, were evaluated. It was concluded that in spite of the fact that fibrous ion exchangers are generally more expensive (approximately 25%) than granular ones, they can improve economics of the waste treatment as:
►In some cases they have higher selectivity for a valuable metal and higher operating capacity
►Due to faster reaction rates, the size of the adsorption unit and, hence, overall treatment plant footprint could be much smaller compared with a resin plant
►The benefits for application of fibrous ion exchange instead of granular material should be quantified and evaluated for each specific case
►Elution instead of incineration might increase the benefits of fibrous materials over granular ones for the waste treatment sequence.
Before designing a process to treat a given PGM waste or low-grade stream, laboratory studies should be performed to optimize the choice of resin, elution system, and operating parameters. In order to do this, it is essential that the chemistry of the metals in the waste stream be understood.
References
Anonymous. Not dated. Equilibriums in solutions of palladium chloride http://www.chemweek.ru/sobitiya/Ravnovesiya_v_rastvorah_kompleksnyh_hloridov_palladiya.htm [in Russian]. [Accessed 12 March 2014]. [ Links ]
Bernardis, F.L., Grant, R.A., and Sherrington, D.C. 2005. A review of methods of separation of the platinum-group metals through their chlorocomplexes. Reactive and Functional Polymers, vol. 65. pp. 205-217. [ Links ]
Boehm, D.R. and Hampel, K.R. 1972. Recovery of palladium from rinse water. US patent 3656939. Gulf & Western Ind. Prod. Co. Publ. 18 Apr.. 1972. [ Links ]
Borg, R.J., Franke, A.A., Nervik, W.E., and Stevenson, P.C. 1955. A method of separating certain platinum group metals with cation exchange resins. US patent 2714555. Publ. 2 Aug. 1955. [ Links ]
Danks, M. Not dated. Smopex® fibres for metal recovery. http://Www.Docstoc.Com/Docs/114085582/Smopex-%EF%BF%BD-Fibres-For-Metal-Recovery [ Links ]
Ginzburg, S.I., Ezerskaya, N.A., Prokof'eva, I.V., Fedorensko, N.V., Schlenskaya, V.I., and Bel'skii, N.K. 1975. Analytical Chemistry of Platinum Metals. Translated by N. Kaner. Shnelnitz, P. (ed.). John Wiley & Sons, New York. [ Links ]
Hubicki, Z., Leszczyήska, M., Lodyga, B., and Lodyga, A 2006. Palladium (II) removal from chloride and chloride-nitrate solutions by chelating ion-exchangers containing N-donor atoms. Minerals Engineering, vol. 19. pp. 1341-1347. [ Links ]
Izatt, S.R., Bruening, R.L., and Izatt, N.E. 2012. Some applications of molecular recognition technology (MRT) to the mining industry. T.T. Chen Honorary Symposium on Hydrometallurgy, Electrometallurgy and Materials Characterisation. Wang, S., Dutrizac, J.E., Free, M.L., Hwang, J.Y., and Kim, D. (eds.). TMS, Warrendale, PA. pp. 51-63. [ Links ]
Klooper, R. 2006. An investigation into the complex formation and potential solvent extraction of Os(IV/III) with N,N-dialkyl-N'-acryl(aroyl)thioureas. Magister Scientie thesis, University of Stellenbosch. [ Links ]
Korkisch, J. 2000. Handbook of Ion Exchange Resins: Their Application to Inorganic Analytical Chemistry. Vol. III. Platinum Metals. CRC Press, Boca Raton, FL 288 pp. [ Links ]
Soldatov, V.S. 2008. Syntheses and the main properties of fiban fibrous ion exchangers. Solvent Extraction And Ion Exchange, vol. 26. pp. 457-513. [ Links ]
Yahorava, V., Kotze, M,. and Auerswald, D. 2013. Evaluation of different adsorbents for copper removal from cobalt electrolyte. Seventh Southern African Base Metals Conference, White River, Mpumalanga, South Africa, 2-6 September 2013. The Southern African Institute of Mining and Metallurgy, Johannesburg. pp. 283-298. [ Links ]
Zaganiaris, E.J. 2009. Ion Exchange Resins in Uranium Hydrometallurgy. Books On Demand, Stoughton, WI. p. 200. [ Links ] ♦














