Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.114 n.2 Johannesburg Feb. 2014
PRECIOUS METALS CONFERENCE 2013
Aspects of coloured precious metal intermetallic compounds
E. van der Lingen
Department of Engineering and Technology Management, Graduate School of Technology Management, University of Pretoria, South Africa
ABSTRACT
This paper provides a review on coloured gold, platinum, and palladium intermetallic compounds, and discusses the models that were developed to obtain these materials. These compounds have a crystal structure of high symmetry, such as the CaF2 or CsCl structures, ensuring distinct electron band structures. Various examples of coloured gold, platinum, and palladium intermetallic compounds are provided. More in-depth discussion is provided on the purple gold (AuAl2) and yellow platinum-aluminium (PtAl2) compounds with CaF2 structure, as well as the purplish-pink palladium-indium (PdIn) compound with CsCl structure.
Precious metal intermetallic compounds are used in jewellery and provide a new dimension to design. Some of these compounds have also found use as barrier coatings on turbine blades for jet engines, and more recently, research has been conducted into their potential use as catalysts, electro-catalysts, sensors, capacitors, and for decorative coatings.
Keywords: colour, gold, platinum, palladium, intermetallic compounds, CaF2 structure, CsCl structure, AuAl2, PtAl2, Pdln.
Introduction
Intermetallic compounds are compounds consisting of two or more metals in which the numbers of the atoms of the different metals are at, or near, a simple ratio e.g. PtAl2. In most cases, intermetallic compounds solidify at a fixed temperature and composition, and have thus a narrow domain of existence. The crystal structure of an intermetallic compound is normally different from those of the individual metals from which it is composed. Fundamental properties of intermetallic compounds are usually high brittleness with associated low toughness, high hardness, good wear resistance, and good corrosion resistance.
Only about 100 compounds among the 30 000 substances in Pearson's Handbook on Crystallographic Data for Intermetallic Phases (Villars and Calvert, 1991) are coloured. Well-known coloured intermetallic compounds are: golden-yellow Cu5Sn; blue NiAl; yellow CoAl; yellow CoGa; blue AuGa2; blue AuIn2; red PdIn; purple AuAl2; blue-grey NiSi2; and dark blue CoSi2.
Colour formation in intermetallic compounds
The formation of colour in metals is based on metallic bonding between different metals. The strong metallic bonds consist of positively charged metal atoms in fixed positions, surrounded by delocalized electrons. Colour results from the electrons in the lower energy levels being excited to higher levels. However, colour in metals can also be formed by intermetallic compounds where strong covalent bonds replace the metallic bonds.
Some models have been developed indicating the requirements for obtaining coloured intermetallic compounds. Brief descriptions of these models are provided here, and if more in-depth scientific support to these models/concepts is needed, the reader can consult the referenced papers. The three models are:
(i) Pettifor's structure maps
(ii) Hume-Rothery electron concentration
(iii) Valence electron concentration.
Pettifor's structure maps
According to Steinemann (Steinemann, 1990; Steinemann et al., 1997; 2002), coloured intermetallic compounds possess a pseudobandgap, which is an energy range with only a few available quantum states, represented by a valley in the density of states curve. These intense localized bands are found approximately 1.5 to 3 eV below the Fermi level. The following three requirements have been identified by Steinemann in order to obtain coloured intermetallic compounds:
(1) The crystal structure of the compound is of high symmetry that has strong features of the band structure, i.e. sharp peaks and valleys in the density of states
(2) Hybrid d-sp bonds for strong covalent hybridization
(3) A late transition element or precious metal shifts the Fermi energy appropriately close to the pseudogap.
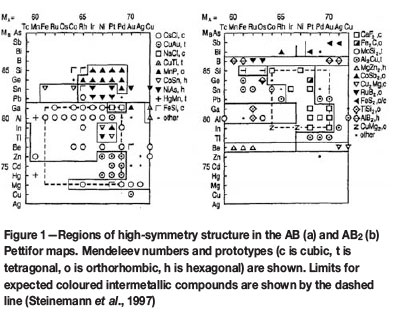
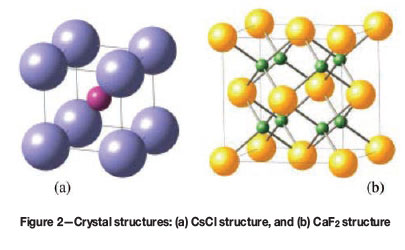
Steinemann et al. (2002) described how it is possible to establish a relationship between colour and crystal structure in intermetallic compounds by making use of Pettifor's structure maps (Pettifor, 1985; 1986; Pettifor and Podlouchy, 1985). Pettifor's structure maps plot crystal structures of binary compounds AxBy of any stoichiometry to a two-dimensional map of some 'coordinates' for elements A and B. Figure 1 shows structure maps for compounds of stoichiometries AB and AB2, which could be candidates for coloured intermetallic compounds. The regions marked with dashed lines reveal potential candidates for binary coloured intermetallic compounds. Interestingly, only two crystal structures (Figure 2) dominate these regions, namely bcc-based B2 (Pearson symbol cP2) or CsCl-structure for composition AB, and fcc-based C1 (Pearson symbol cF12) or CaF2-structure for composition AB2. This again confirms the three requirements stipulated above that coloured intermetallic compounds need to have a crystal structure of highest symmetry, ensuring a sufficiently simple electronic structure for distinct absorption bands of high intensity. According to this approach, only the following potential coloured binary gold, palladium, and platinum intermetallic compounds can result:
CsCl structure: PdIn, PdBe, PdMg
CaF2 structure: PdAl2, PtSn2, PtGa2, PtAl2, PtIn2,
AuGa2, AuAl2, AuIn2.
Element B is of Groups 13 and 14 in the Periodic Table, with the exception of PdMg. Furthermore, PtAl (tetragonal structure) also exhibits colour according to Figure 1 (Steinemann et al., 1997). Interestingly, PtGa and PdGa, which have a B1 structure, but do not exhibit colour, are omitted due to a missing pseudogap. PtMg also does not reveal colour as its B20 structure, although cubic, is of lower symmetry. PtGa2 andPtIn2 are stable only at temperatures above 153°C and 674°C, respectively (Steinemann, 1990).
Hume-Rothery electron concentration
An electron-to-atom ratio is stipulated for the Hume-Rothery phases, where the electron concentration (e/a) is defined as the sum of the valence electrons per atom of the compound:
e/α = 1/100 - Σαiνi
where
ai is the concentration in at.%
νi is the number of valance electrons of element i.
The number of valence electrons for platinum and palladium is 0 according to Ekman's rule, and valence electron numbers are 1, 2, and 3 respectively for Groups 1, 2, and 13 (Al, Ga, In) (Steinemann et al., 2002). Table I gives the specific values for the elements and compounds.

Accordingly, the CsCl structure is stable when e/a is approximately 1.1 to 1.7, for example PdIn. Furthermore, the CaF2 structure is stable if e/a is approximately 2.0 to 2.67 as in the case of PtAl2, PtGa2, and PtIn2.
The valence electron concentration
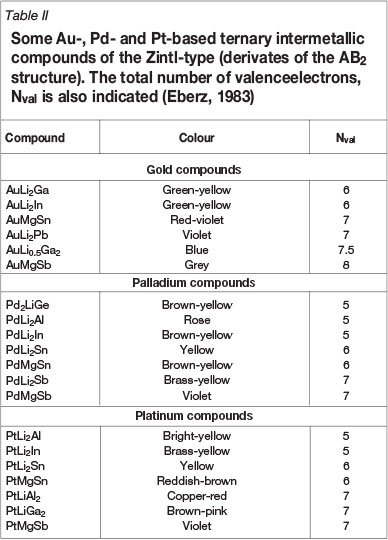
An extension of the Hume-Rothery electron concentration model is the upper limit on the valence electron concentration according to Schlemper and Thomas (1994), which applies to the Zintl phases, for more complex ternary and quaternary compounds. The high number of valance electrons of the precious metals determines the appropriate location of the Fermi level inside the pseudogap, providing absorption bands for creating colour. Eberz (1983) indicated that only intermetallic compounds with valence electron numbers equal to or smaller than about 7 will exhibit colour. Some examples are provided in Table II for various Au, Pd, and Pt compounds (Eberz, 1983).
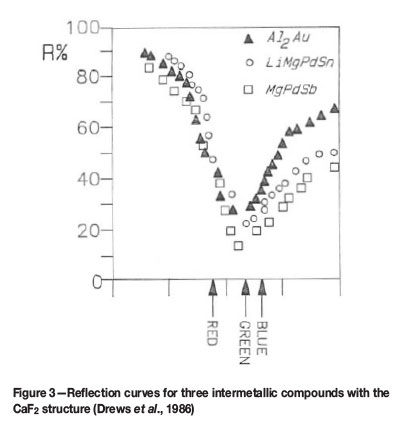
Drews et al. (1986) have published interesting results on the optical properties and structures of a number of ternary and quaternary compounds containing platinum or palladium. These compounds are of type LixMgyPS, where P is palladium or platinum and S is tin (Sn) or antimony (Sb). Sometimes x=0, in which case one has a ternary compound. The reflection spectra of all these compounds are similar, indicating colours ranging from yellow to purple, e.g. LiMgPdSn (violet); LiMgPtSn (bright red); LiMgPtSb (violet) etc. By varying the relative amounts of Li and Mg in Li2-xMgxPdSn (where x can vary from 0 to 1), Drews et al. found that the lattice parameter increased by 1.5% with a colour change from yellow to copper-red and then to red-violet. Figure 3 shows the amount of light reflected as a function of colour (wavelength) for three different intermetallic compounds. The valley in the reflection curve near the green portion of the spectrum leads to stronger reflection at the blue and red ends of the spectrum, resulting in a pink or purple colour. The sharp rise in reflectivity in the blue part of the spectrum is most likely due to increased absorption, resulting from a hybrid resonance between d-electrons of a noble metal and conduction electrons.
Applications
Jewellery
The three main colours of caratage gold alloys, namely yellow, red, and white, are well known. The less-known colours of gold include blue, purple, and black. A review of coloured gold alloys was published by Cretu and Van der Lingen (1999, 2000). Coloured gold alloys can be produced by three metallurgical routes:
(i) Alloying with elements such as copper, which results in a more reddish colour; or silver, giving a more white-greenish colour.
(ii) Coloured oxide layer formation by alloying with an oxidizing element, such as iron, and exposing the alloy to an oxidizing heat treatment.
(iii) Intermetallic compounds, which are addressed in the present review.
The most popular coloured intermetallic gold compound is purple AuAl2, which is formed at a composition of 79 wt%Au and 21 wt%Al. This material can be hallmarked as 18 carat gold, which requires at least 75 wt% gold. Due to the brittleness of intermetallic compounds, jewellers have used the colourful compound as inlays, gemstones, and in bi-metal castings (Figure 4). The melting point of AuAl2 is 1060°C.

Two other intermetallic compounds that are known to produce colours in gold alloys, as also revealed by Pettifor's structure maps, are AuIn2 and AuGa2. The gold-indium intermetallic compound AuIn2 has a clear blue colour and forms at 46 wt%Au, and AuGa2 at 58.5 wt%Au has a slight bluish hue. The latter compound can be hallmarked as 14 carat gold. The reflectivity falls in the middle of the visible spectrum and rises again towards the violet end, giving distinctive colours in each case (Vishnubhatla and Jan, 1967). Figure 5 shows the reflectivity as a function of the energy of the incident light for AuAl2, AuIn2, and AuGa2 (Saeger and Rodies, 1977).

The inherent brittleness of the coloured gold intermetallic compounds can be improved by micro-alloying additions (<2 wt%), such as additional aluminium, palladium, copper, or silver (Wongpreedee, 2006).
Platinum intermetallic compounds
Unlike gold, platinum and palladium have a strong white lustre and act as bleaching agents, making them very difficult to colour by conventional alloying as in the case of gold. Both coloured gold and platinum intermetallic compounds have the CaF2 structure with alloying elements X = Al, In, and Ga. Klotz (2010) found that interesting colour effects can be achieved by an exchange of gold with platinum while keeping a constant atomic ratio of (Au,Pt)X2. For blue gold, increasing platinum content changes the blue AuIn2 colour towards apricot PtIn2.
Mintek in South Africa has found that two distinct colours, namely orange and pink, result by adding different amounts of copper to the PtAl2 compound (Hurly, 1991; Hurly and Wedepohl, 1993). The optimum compositions for the colours are shown in Table III.
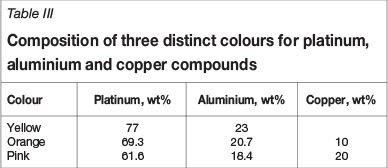
Figure 6 shows the measured CIELab colour co-ordinates (only positive a* and b* values) for the three samples in Table III, as well as unalloyed platinum, gold, silver, and some standard gold alloys. The a* co-ordinate is a measure of the intensity of the red and green colours of the sample: an increasingly positive a* indicates more red in the sample, and increasingly negative a* values indicate more green. Similarly, b* measures yellow and blue: increasingly positive b* indicates more yellow, and increasingly negative b* more blue (ASTM-E308-01, 2001).

Figure 7 shows percentage reflectivity as a function of wavelength for PtAl2 and other samples containing varying amounts of copper. An increase in the copper content results in a change of colour from the characteristic brass-yellow of PtAl2 through orange to pink. The sample containing 25% copper has a minimum in the green region of the spectrum (about 500 nm), and the higher reflectivities at the blue and particularly red ends of the spectrum combine to give the characteristic pink colour.

Hurly and Wedepohl (1993) found from X-ray diffraction studies of PtAl2 with various copper additions that all the samples (up to 25 wt% Cu) had the basic fluorite structure (CaF2) of PtAl2. The lattice parameter increased with copper content as the colour changed. For PtAl2 with 25wt% copper, the lattice parameter is about 0.8% greater than that of pure PtAl2.
As with purple gold, coloured platinum intermetallic compounds lend themselves to be treated like gemstones and can be facetted by using standard gem cutting equipment and techniques. Figure 8 shows a jewellery item with facetted pink-coloured platinum compounds, also known as Platigem®.

Palladium intermetallic compounds
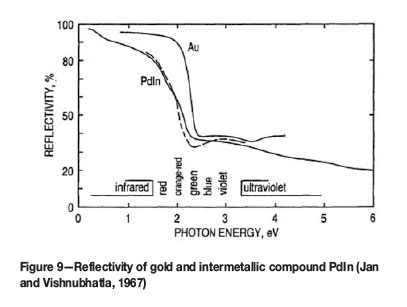
According to the binary In-Pd phase diagram (Massalski et al., 1986), five In-Pd intermetallic compounds exist, namely In3Pd, In3Pd2, InPd, InPd2, and InPd3. The In-Pd intermetallic compound with composition 50 at.% (48 wt%) palladium and 50 at.% (52 wt%) indium has a purplish-pink colour. Jan and Vishnubhatla (1967) investigated optical properties and Harris et al. (1968) investigated structures of palladium-indium alloys. Figure 9 shows results of optical measurements for gold and PdIn. More than 95% of incident light is reflected by gold in the infrared and longer wavelength range of visible light. At energies higher than 1.9 eV, the reflectivity falls off rapidly with diminishing wavelength. The yellow colour of gold results from its strong absorption of light above energies of about 2.3 eV. The metal reveals the complementary colour of the absorbed frequencies. With PdIn, the absorption occurs at lower energies, and the colour of the compound then appears as purplish pink, also confirmed by Nomerovannaya et al. (1979). The absorption above about 2.2 eV is associated with a large loss function and interband transitions of hybridized states around the Fermi energy (Cho, 1970). The corresponding CIELab values for PdIn are L* = 70, a* = +12', b* = +6.
Schaffer and Ingersoll (1989) and Steinemann (1990) have investigated the effect of different alloying elements on the PdIn system. A summary of the results is compiled in Table IV.
Coatings
PtAl2
Platinum-modified aluminide coatings have been used for several decades as diffusion barrier coatings in aircraft and industrial gas turbines (Pomeroy, 2005). These coatings provide improved resistance to both high-temperature oxidation and hot corrosion. Hot corrosion occurs in gas turbines due to the presence of contaminants such as NaCl, Na2SO4, and V2O5 in the gases, which form molten deposits that damage the turbine blades (Rajendran, 2012).
The platinum-modified nickel aluminide coatings can exist in two forms depending on how they were formed. Figure 10 shows the two forms, where (a) indicates the two phase PtAl2 + (Ni-Pt-Al), and (b) a single-phase (Ni-Pt-Al) coating. Platinum is initially deposited onto the nickel-based superalloy by electroplating, then heat-treated under a protective atmosphere. The heat treatment conditions influence the formation of a single- or two-phase microstructure. Subsequent aluminizing results in the platinum-modified NiAl coating. Figure 11 indicates a depth profile of the platinum-modified NiAl coating in Figure 10 (a).


The advantages that platinum offers in barrier diffusion coatings can be summarized as follows (Rajendran, 2012 and references therein). Platinum:
►Improves the high-temperature oxidation resistance by delaying transformation of β-NiAl into y'-Ni3Al in aluminides. The life of the diffusion coating is depleted when all β-NiAl has transformed into y'-Ni3Al.
►Acts as a catalyst promoting the reaction between aluminium and oxygen.
►Improves the adhesion between the coating and substrate.
►Suppresses deleterious spinel formation.
►Retards the diffusion of certain refractory elements to the coating-Al2O3 interface, providing improved isothermal oxidation resistance.
An excellent review by Das (2013) provides information on the importance of the platinum and aluminium contents for the microstructure and oxidation performance of the modified platinum-aluminide bond coatings.
AuAl2, AuIn2, and AuGa2
Supansomboon et al. (2008) prepared AuAl2 coatings by vacuum deposition onto heated substrates. The coloured coatings varied in colour from dark silver to light purple, whereas the transmission colours of these coatings varied from light to dark greenish-brown. The colour observed by the human eye was dependent on the texture of the substrate, and the crystallized microstructure and the coating thickness affected the transmission colours. The potential use of AuAl2 as a spectrally selective coating on architectural glass was explored, but the compound was found to be inferior to gold in terms of selective attenuation of the infrared radiation. Furrer et al. (2013) found that the light purple colour of AuAl2 coatings is due to point defects in the film resulting from the deposition method. The intense purple colour can be obtained by heat treating the coating at 350°C.
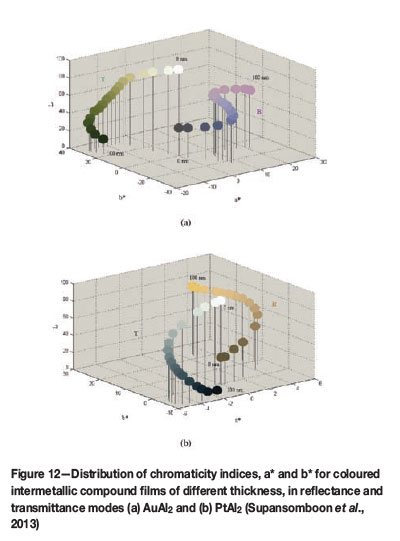
Supansomboon (2013) produced AuAl2 and PtAl2 coatings by a co-sputtering technique, and similar X-ray diffraction patterns were obtained to those of the bulk AuAl2 and PtAl2 samples. Figure 12 shows the CIELab colour measurements, in both reflectance and transmittance mode, for AuAl2 and PtAl2 coatings of different thicknesses. Potential uses include decorative applications and jewellery.
Studies by Keast et al. (2013) indicated that PtAl2 and AuAl2 coatings have dielectric functions suitable for sustaining localized plasmon resonances, as verified with EELS and reflectivity measurements. The results suggested that the PtAl2 compound is a better candidate for the development of strong localized surface plasmon resonances compared to AuAl2.
In a project funded by the European Commission on surface engineering of the colour effect for gold alloys, Klotz (2010) found that the electroplating/annealing process was very successful for producing AuIn2 layers, whereas surface cladding worked well for both AuGa2 and AuIn2, and liquid metal dip-coating for AuGa2 (Figure 13).

PdIn
Wang et al. (1990) developed a thermally stable, low-resistance PdIn ohmic contact to n-GaAs. A layer of In0.4Ga0.6As approximately 5 nm in thickness covered the interface between the single-phase intermetallic PdIn layer and the GaAs substrate. Specific contact resistivities and contact resistances of approximately 1x10-6 ω cm2 and 0.14 ω mm respectively, were obtained.
Catalysis, sensors, and capacitors
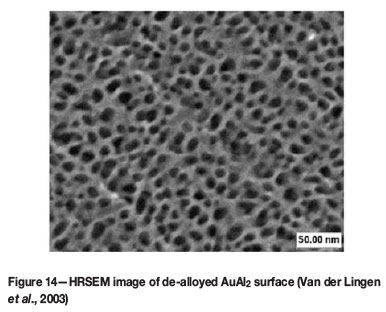
Mesoporous, also known as nanoporous, gold and platinum can be prepared by de-alloying AuAl2 and PtAl2 in a manner analogous to that used in the preparation of Raney nickel catalysts (Van der Lingen et al., 2001). The aluminium is then dissolved from the AuAl2 precursor by means of NaOH to produce a highly porous gold structure, (Figure 14). Van der Lingen et al. (2003) produced heterogeneous catalysts by incorporating transition metal oxide(s) with the porous gold. Furthermore, promoter elements could also be melted with AuAl2 to improve catalytic activity. These catalysts were tested for CO oxidation activity.
Pattrick et al. (2003) studied mesoporous gold catalysts prepared by de-alloying of AuAl2 for the selective catalytic reduction of NOx by propene under lean-burn conditions for potential autocatalyst applications. It was found that relatively low additions of platinum group metals (1 at.%) caused shifts to lower temperature regions of activity, with the largest shift obtained for rhodium, most likely due to a strong synergistic interaction between rhodium and gold.
Cortie and Van der Lingen (2003) investigated the potential of mesoporous gold for ultra-capacitors, and preliminary work revealed a perceived capacitance of about 15-28 mF as demonstrated for a sample containing 2 g of gold when connected as a cathode. No storage of energy was observed when the gold was connected as the anode. Further work in this field by Mortari et al. (2007) on de-alloyed gold indicated that the electrodes showed near-ideal capacitor behaviour under both cyclic voltammetry and potential-step conditions. They proposed that the mesoporous gold electrodes could offer a convenient way to sensitively and accurately amplify the capacitance signal of an electrochemical sensor.
A significant number of papers have been published recently on nanoporous gold, but the precursor material is 30 wt% gold and 70 wt% silver. A similar de-alloyed structure is obtained for the gold-silver system as for the AuAl2 system. Potential applications for the nanoporous gold includes: catalysis for oxygen-assisted coupling reactions (Stowers et al., 2013); CO oxidation (Röhe et al., 2013); and electro- catalysis/fuel cells (Jin et al., 2013); electrochemical immunoassays and sensors (Sun et al., 2013; Zhu et al., 2013; Li et al., 2013).
Rameshan (2012) studied the formation and thermo-chemical and catalytic properties of Pd-In near-surface intermetallic phases and correlated the findings to those from the PdZn and PdGa systems. The multilayer PnIn intermetallic phase yielded a highly CO2-selective catalyst for methanol steam reforming, although it was not very active in the temperature range 493-623 K. However, in an In-diluted PdIn intermetallic phase, which correlated with the PdZn system, CO2 formation was largely suppressed and CO formation enhanced via full methanol dehydrogenation.
Conclusions
Coloured precious metal intermetallic compounds display a range of colours varying from yellow to violet, depending on the specific composition. These coloured compounds have CaF2 or CsCl crystal structures. The most well-known precious metal intermetallic compounds with CaF2 structure are purple AuAl2 and yellow PtAl2. Copper additions to PtAl2 can further result in a colour variation from yellow to orange to pink. In jewellery applications these compounds can be used as gemstones, coatings, or inlays in combination with yellow carat gold or white platinum jewellery alloys. The PdIn compound with CsCl crystal structure has a distinct purplish-pink colour, and studies on the effect of different alloying elements on various properties have shown, for example, that additions of up to 20 wt% of other precious metals (Pt, Rh, Ru, Ir) can decrease the thermal expansion coefficient and act as grain refining elements.
Coloured precious metal intermetallic compounds are also used as turbine blade coating material where resistance to high-temperature oxidation and hot corrosion attack is required. The use of coloured precious metal intermetallic compounds as decorative coatings on glass supports is still in the development phase and no commercial products are yet available. De-alloying of precious metal intermetallic compounds can result in mesoporous, also known as nanoporous, materials. These materials have relatively high surface areas, which lend them to further development as catalysts, electro-catalysts, sensors, and capacitors.
Precious metal intermetallic compounds are used in various applications. Although they are inherently brittle, which could restrict their use, this limitation is overcome by using the compounds in powder form, coatings, and inlays with bi-metallic castings.
References
ASTM: E308-01. 2001. Standard Practice for Computing the Colors of Objects by using the CIE System. American Society for Testing and Materials (ASTM). [ Links ]
Cho, S.J. 1970. Energy bands in β'-PdIn. Physica Status Solici, vol. 41, no.1. pp.179-189. [ Links ]
Cortie, M.B. and Van Der Lingen, E. 2003. Properties and potential applications of meso-porous gold. Gold 2003. New Industrial Applications/or Gold. World Gold Council and Canadian Institute of Mining, Metallurgy and Petroleum: Vancouver, Canada [ Links ]
Cretu, C. and Van Der Lingen, E. 1999. Coloured gold alloys. Gold Bulletin, vol. 34, no. 2. pp. 115-124. [ Links ]
Cretu, C. and Van Der Lingen, E. 2000. Coloured gold alloys. Gold Technology, vol. 30. pp. 31-40. [ Links ]
Das, D.K. 2013. Microstructure and high temperature oxidation behavior of Pt-modified aluminide bond coats on Ni-base superalloys. Progress in Materials Science, vol. 58. pp. 151-182. [ Links ]
Drews, J., Eberz, U., and Schuster, H-U. 1986. Optische Untersuchungen an farbigen Intermetallischen phasen. Journal of the Less-Common Metals, vol. 116. pp. 271-278. [ Links ]
Eberz, U. 1983. Fabrige tenäre und quaternäre intermetallische phasen mit platinmetallen - Zintl - phasen. PhD dissertation, University of Cologne. [ Links ]
Fischer-Buhner, J., Basso, A., and Poliero, M. 2010. Metallurgy and processing of coloured gold intermetallics - Part II: Investment casting and related alloy design. Gold Bulletin, vol. 43, no. 1. pp. 11-20. [ Links ]
Furrer, A., Seita, M., and Spolenak, R. 2013. The effects of defects in purple AuAl2 thin films. Acta Materialia, vol. 61. pp. 2874-2883. [ Links ]
Harris, I.R., Norman, M., and Bryant, A.W. 1968. A study of some palladiumindium, platinum-indium and platinum-tin alloys. Journal of the Less-Common Metals, vol. 16, no. 4. pp. 427-440. [ Links ]
Hurly, J. and Wedepohl, P.T. 1993. Optical properties of coloured platinum intermetallic compounds. Journal of Materials Science, vol. 28. pp. 5648-5653. [ Links ]
Hurly, J. 1991. Intermetallic Compounds. US Patent 5,045,280, Sep. 3 1991 [ Links ]
Jan, J-P. and Vishnbhatla, S.S. 1967. Optical properties of the beta-phase alloys AuZn, GaZn and PdIn. Canadian Journal of Physics, vol. 45, no. 8. pp. 2505-2511. [ Links ]
Jin, W., Liu, J., Wang, Y., Yao, Y., Gu, J., and Zou, Z. 2013. Direct NaBH4-H2O2 fuel cell based on nanoporous gold leave. International Journal of Hydrogen Energy, vol. 38, no. 25. pp. 10992-10997. [ Links ]
Keast, V.J., Zwan, B., Supansomboon, S., Cortie, M.B., and Persson, P.O.A. 2013 AuAl2 and PtAl2 as potential plasmonic materials. Journal of Alloys and Compounds, vol. 577. pp. 581-586. [ Links ]
Klotz, E.U. 2010. Metallurgy and processing of coloured gold intermetallics -Part I: Properties and surface processing. Gold Bulletin, vol. 43, no 1. pp. 4-10. [ Links ]
Li, M., Zhang, M., Ge, S., Yan, M., Yu, J., Huang, J., and Liu, S. 2013. ultrasensitive electrochemiluminescence immunosensor based on nanoporous gold electrode and Ru-AuNPs/graphene as signal labels. Sensors and Actuators B: Chemical, vol. 181. pp. 50-56. [ Links ]
Massalski, T., Murray, J., Lawrence, B., and HUGH, B. (eds.) 1986. Binary Alloy Phase Diagrams. American Society of Metals, Metals Park, Ohio. [ Links ]
Mortari, A., Maaroof, A., Martin, D., and Cortie, M.B. 2007. Mesoporous gold electrodes for sensors based on electrochemical double layer capacitance. Sensors and Actuators B: Chemical, vol. 123, no. 1. pp. 262-268. [ Links ]
Nomerovannaya, L.V, Kirillova, M.M., Savitskii, E.M., Polyskova, V.P., Gorina, N.B., and Keronovskii, N.L. 1979. The origin of the colour of PdIn intermetallic compound. Soviet Physics Doklady, vol. 24, no. 5. pp. 379-382. [ Links ]
Pattrick, G., Van Der Lingen, E., Schwarzer, H., and Roberts, S.J. 2003. Development of gold-sponge catalysts for lean-burn DeNOx. Gold 2003. New Industrial Applications for Gold. World Gold Council and Canadian Institute of Mining, Metallurgy and Petroleum, Vancouver, Canada [ Links ]
Pettifor, D.G. and Podlouchy, R. 1985. A microscopic theory of the structural stability of pd bonded AB compounds. Physics Review Letters, vol. 53, no. 2. pp. 1080-1083. [ Links ]
Pettifor, D.G. 1986. New alloys from the quantum engineer. New Scientist, vol. 110, no. 1510. pp. 48-53. [ Links ]
Pettifor, D.G. 1985. Intermetallic Compounds - Principles, vol. 1. Westbrook, J.H. and Fleischer, R.L. John Wiley & Sons, UK. pp. 419-438. [ Links ]
Pomeroy, M.J. 2005. Coatings for gas turbine materials and long term stability issues. Materials and Design, vol. 26. pp. 223-231. [ Links ]
Rajendran, R. 2012. Gas turbine coatings - an overview. Engineering Failure Analysis, vol. 26. pp. 355-369. [ Links ]
Rameshan, C., Lorenz, H., Mayr, L., Penner, S., Zemlyanov, D., Arrigo, R., Haevecker, M., Blume, R., Knop-Gericke, A., Schlogl, R., and Klotzer, B. 2012. CO2-selective methanol steam reforming on In-doped Pd studied by ambient-pressure X-ray photoelectron spectroscopy. Journal of Catalysis, vol. 295, no. 2-3. pp. 186-194. [ Links ]
Rohe, S., Frank, K., Schaefer, A., Wittstock, A., Zielasek, v., Rosenauer, A., and BAumer, M. 2013. CO oxidation on nanoporous gold: A combined TPD and XPS study of active catalysts. Surface Science, vol. 609. pp. 106-112. [ Links ]
Saeger, K.E. and Rodies, J. 1977. The colour of gold and its alloys. Gold Bulletin, vol. 10, no. 1. pp. 10-14. [ Links ]
Schaffer, S. P. and Ingersoll, C.E. 1989. Gold coloured palladium indium alloys. US Patent 4,804,517. 14 Feb, 1989. [ Links ]
Schlemper, K. and Thomas, L.K. 1994. Optical properties and electronic structure of the intermetallic phases NiAl, CoAl, and FeAl. Physics Review B, vol. 50, no. 24. pp. 17802-17810. [ Links ]
Steinemann, S.G., Anongba, P.N.B., and Podloucky, R. 1997. Color in Pettifor's structure maps: Intermetallic compounds for a new use. Journal of Phase Equilibria, vol. 18, no. 6. pp. 655-662. [ Links ]
Steinemann, S.G. 1990. Intermetallic compound, method for producing the compound, and use of the compound. US Patent 4,911,762. Mar. 27 1990 [ Links ]
Steinemann, S.G., Wolf, W., and Podloucky, R. 2002. Colour and Optical Properties, vol. 3. Intermetallic Compounds -Principles and Practise. Westbrook, J.H. and Fleischer, R.L. (eds.). John Wiley & Sons, UK. pp. 231-244. [ Links ]
Stowers, K.J., Madix, R.J., and Friend, C.M. 2013. From model studies on Au(111) to working conditions with unsupported nanoporous gold catalysts: Oxygen-assisted coupling reactions. Journal of Catalysis, vol. 308. pp. 131-141. [ Links ]
Sun, G., Lu, J., Ge, S., Song, X., Yu, J., Yan, M., and Huang, J. 2013. ultrasensitive electrochemical immunoassay for carcinoembryonic antigen based on three-dimensional macroporous gold nanoparticles/graphene composite platform and multienzyme functionalized nanoporous silver label. Analytica Chimica Acta, vol. 775. pp. 85-92. [ Links ]
Supansomboon, S., Maarool, A., and Cortie, M.B. 2008. Purple Glory: The optical properties and technology of AuAl2 coatings. Gold Bulletin, vol. 41, no. 4. pp. 296-304. [ Links ]
Supansomboon, S., Dowd, A., Van Der Lingen, E., and Cortie, M.B. 2013. Coatings of coloured intermetallic compound for decorative and technological applications. Materials Innovation in Surface Engineering (MISE) 2013 Conference, Adelaide, Australia, 19-21 November 2013. [ Links ]
Van Der Lingen, E., Cortie, M.C., and Glaner, L. 2001. Catalyst and method of producing a catalyst. SA Patent Application no. 2001/5816. [ Links ]
Van Der Lingen, E., Cortie, M.B., Schwarzer, H., Roberts, S.J., Pattrick, G., and Compton, D. 2003. Gold catalysts prepared via intermetallic precursor. Gold 2003. New Industrial Applicationsfor Gold.. World Gold Council and Canadian Institute of Mining, Metallurgy and Petroleum, Vancouver, Canada [ Links ]
Villars, P. and Calvert, L.D. 1991. Pearson's Handbook of Crystallographic Data for Intermetallic Phases. ASM International, Materials Park, Ohio. [ Links ]
Vishnubhatla, S.S. and Jan, J.P. 1967. Optical properties of the intermetallic compounds AuAl2, AuGa2 and AuIn2. Philosophical Magazine, vol. 16, no. 39. pp. 45-50. [ Links ]
Wang, L. C., Wang, X. Z., Lau, S. S., Sands, T., Chan, W. K., and Kuech, T. F. 1990. Stable and shallow PdIn ohmic contacts to n-GaAs. Applied Physics Letters, vol. 56, no. 21. pp. 2129-2131. [ Links ]
Wongpreedee, K. 2006. Purple gold: past, present, and future to ductile intermetallics. Gold 2006, University of Limerick, Ireland. World Gold Council. [ Links ]
Zhu, X-T., Zhang, L-J., Tao, H., and Di, J-W. 2013. synthesis of nanoporous gold electrode and its application in electrochemical sensor. Chinese Journal of Analytical Chemistry, vol. 41, no. 5. pp. 693-697. [ Links ] ♦