Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Journal of the Southern African Institute of Mining and Metallurgy
On-line version ISSN 2411-9717
Print version ISSN 2225-6253
J. S. Afr. Inst. Min. Metall. vol.113 n.12 Johannesburg Jan. 2013
GENERAL PAPERS
A study on the effect of coke particle size on the thermal profile of the sinters produced in Esfahan Steel Company (ESCO)
A. DabbaghI; A. Heidary MoghadamII; S. NaderiI; M. HamdiI
ICentre of Advanced Manufacturing and Materials Processing, University of Malaya, Kuala Lumpur, Malaysia
IIMetallurgy Department, Engineering Faculty, Islamic Azad University, Dezfool, Iran
SYNOPSIS
The aim of this research was to investigate the effect of coke particle size on the thermal profile of the charge bed in the sintering process of iron ore. Six different ranges of coke particle size that could be used in practice in the sintering line of Esfahan Steel Company (ESCO) were used while parameters such as ratios and particle sizes of other ingredients, ignition temperature, and suction power were kept constant. Thermal profiles were obtained by measuring the temperature changes at three points in the sintering bed. The influence of coke particle size on rate of temperature rise, maximum temperature of the sintering bed, and the flame front velocity of the sintering bed was determined by analysing the thermal profiles. Moreover, the cold strengths of the sinters were measured using the Tumbler method. The results indicated that the coke particle size has a significant impact on the thermal properties of the sintering bed. Based on the results, a coke particle size between 0.212 mm and 3.350 mm was determined as the optimal range for the specific conditions in the sintering line of ESCO.
Keywords: iron ore sintering, coke particle size, thermal profile, flame front velocity, Tumbler index.
Introduction
The sintering of iron ore is an important stage in the steelmaking process. In this procedure, the temperature rise due to the combustion of coke breeze causes the partial fusion and diffusion bonding in the sintering charge, producing a coarse agglomerate suitable for charging in the blast furnace (Ramos et al., 2000; Umadevi et al., 2011). The sinters produced must simultaneously possess high mechanical strength and porosity (Raygan et al., 2009; Yang et al., 2004). It is confirmed by experience that any change in the chemical and physical conditions during the sintering process could significantly affect the blast furnace efficiency in different aspects, especially energy consumption and total duration of the steelmaking process (Loo, 2005; Umadevi et al., 2011). Among various parameters governing the sintering process, the thermal profile along the sintering bed has a significant impact on the quality of the produced sinter. The thermal profile of the sintering bed is generally governed by the flame front velocity and gas flow through the bed (Umadevi et al., 2011); in turn, the flame front properties are strongly affected by coke distribution along the bed as well as the coke particle size (Loo and Wong, 2005). Coke is the major source of energy in the iron ore sintering process, and hence its particle size distribution has a crucial influence on the sintering productivity. The coarse coke particles in the sintering mixture result in a heterogeneous thermal profile along the charge bed. Furthermore, the higher temperatures near the coarse particles might produce an undesired amount of molten phases, which will further decrease the bed diffusivity. On the other hand, too rapid combustion of fine coke particles results in a thin flame front layer, which reduces the productivity of the sintering process. Moreover, excessively fine coke particles could decrease the diffusivity of the bed and thus reduce the airflow rate through the sintering charge (Teo et al., 1992).
A number of studies have investigated the effect of coke particle size on various properties of the iron ore sinters. Kasai et al. (1989) examined the effect of both unsized and sized coke particles on the permeability of the bed prior to ignition and during sintering. They found that the large particles of coke provide an improved permeability before and during the sintering. Teo et al. (1992) reported that the large coke and flux (limestone and serpentine) particles (+1.0 mm) could not effectively attach to the fine particles during granulation. They also stated that coke is a poor granulating material due to its hydrophobic surface properties. Hosotani et al. (1996) found that simultaneous removal of the -1 mm limestone and the -0.5 mm coke breeze fractions improves the granulation index and permeability of the green sinter bed. This led to a suppression in the sinter shrinkage, increase of the sintering rate (and therefore productivity), as well as improved reducibility due to an increase in the number of fine pores. Similar results were also reported by Lwamba and Garbers-Craig (2005). Bhagat et al. (2007) showed that the microporosity and reducibility of the sinters increased by lowering the coke particle size. More recently, Mohamed et al. (2010) proposed a coke particle size between 1 mm and 3 mm as the most favourable range in the sintering process. Differences in the results of these studies are a result of the varied experimental conditions, including the composition, weight ratios, particle size distribution of the other raw materials, and sintering bed geometry.
Despite these extensive studies concerning the various effects of coke particle size, the impact of this parameter on the thermal behaviour of the sintering bed has not been comprehensively investigated. Therefore in this study, the effect of coke particle size on various thermal properties of the sintering bed, including temperature rise rate, maximum temperature of the sintering bed, and flame front velocity was investigated. The cold strength of the produced sinters was also measured using the Tumbler method and, based on the obtained results, the optimal coke particle size suitable for the specific conditions of ESCO's sintering line was specified.
Experimental method
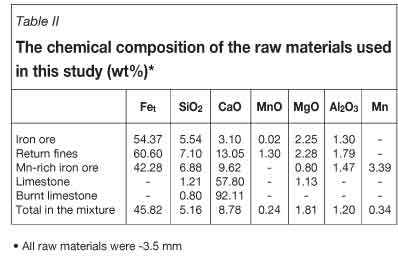
The raw materials used in this research were obtained from ESCO (Table I). The chemical analyses of the raw materials as well as the coke used to prepare sinters are given in Tables II and III, respectively. In the present study, six different ranges of coke particle size which could be used in the sintering line of ESCO were chosen and a particle size of coke that was randomly collected from the ESCO sintering line was considered as reference.


The size distributions of the coke particles selected in this study are shown in Table IV. The particle sizes and weight ratios of the other raw materials used in the experiments were similar to the ESCO sintering charge.
Figure 1 illustrates the experimental set-up used in this study. The inner wall of the sintering pot was covered with a layer of refractory brick (5 cm thickness) to protect the outer steel wall from deformation. The upper surface of the sintering machine was also covered during the sintering process for precautionary purposes. For production of sinters, the coarse particles of all raw materials (+3.5 mm) were firstly removed from the charge, and then a mixture of iron ore, return sinter, burnt limestone, raw limestone, manganese-rich iron ore, and coke was blended for five minutes.
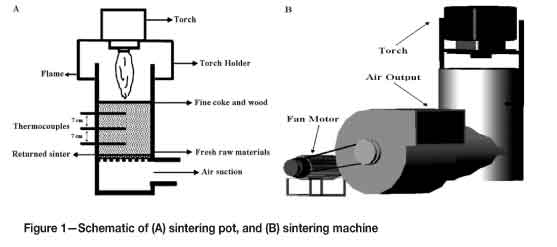
During the blending, two steel balls were placed in the mixer in order to prevent the granulation of raw materials. In each experiment, a sub-layer (3 cm height), which was composed of returned materials from former sintering processes, was placed in the bottom of the sintering pot. Then, a layer (21 cm height) of fresh raw materials was poured on the sub-layer surface. The surface of the second layer was later covered by fine coke powder (-0.5 mm) to facilitate the ignition. Temperature changes along the bed were measured by three thermocouples placed at the bottom, middle, and upper sections of the pot. In order to initiate the sintering process, the layer of fine coke on the surface was ignited by a torch at temperatures near 800°C and the ignition was continued until the higher thermocouple showed an increase in the temperature of the first layer (about 7 minutes for CPS3, 15 minutes for CPS1, and 10 minutes for other specimens). The whole sintering process, from ignition of the coke layer until cooling of sinters to the room temperature, lasted about 60 minutes (except for CPS1 which needed 90 minutes for completion). It is important to note that the exhaust air of the sintering machine possesses a high temperature level and carries some fine particles that might cause serious health hazards. Therefore, the sintering machine was equipped with an exhaust system to direct the air flow away from the critical areas.
Different thermal parameters including temperature rise rate, achieved maximum temperature, and flame front velocity were quantified by analysing the thermal profiles of the samples. The cold strengths of the produced sinters were also measured by the Tumbler method according to the procedure described in ASTM E2 79-97. All experiments were performed at least five times and the results were analyzed by ANOVA with 95% confidence interval using SPSS 20 (SPSS Inc., Chicago, IL). The microstructures of the produced sinters were also investigated using a metallurgical microscope (Olympus, model BX60M, Japan).
Results and discussion
Figure 2 shows the typical thermal profiles achieved in different samples.

According to this figure, the shortest time needed for completion of the sintering process is observed in the CPS3 specimen. In addition, the time intervals between the maximum temperatures of the bottom, middle, and upper thermocouples for CPS3 are shorter than those for the other specimens. Figure 3 shows a schematic of the method applied for calculation of temperature rise rate, maximum temperature, and flame front velocity using the achieved thermal profiles. The mean values of the temperature rise rate, maximum temperature, and flame front velocity obtained for different specimens are plotted in Figure 4a-c, respectively. Figure 4d also shows the changes in the Tumbler index (TI) of the produced sinters with different coke particles sizes.


Temperature rise rate
The rate of temperature rise (r) in the sintering bed is directly correlated with the productivity of the sintering process. In this study, we considered the r value as the rate of temperature rise at the middle thermocouple (Figure 2). Since coke is the only source of energy in the sintering process, the slopes of the thermal curves depend primarily on the coke reactivity as well as the amount of oxygen available for combustion. With a decrease in the coke particle size, the specific surface and thus the reactivity and combustion ability of coke is increased. As a result, the rate of energy production is increased, causing a more rapid rise of temperature in the sintering bed. However, the burning rate of too-fine coke particles is excessively high, which results in a narrow thickness of the high-temperature layer and consequently, a slower temperature rise in the charge bed. In addition to the coke reactivity, the availability of sufficient oxygen is another factor that has a significant impact on the temperature rise rate. In turn, the amount of oxygen at the combustion zone depends primarily on the diffusivity of the charge bed. By elimination of the large coke particles, diffusivity of the charge bed is decreased, providing less oxygen than the stoichiometric amount needed for complete combustion of coke. Therefore, there is a trade-off between the coke reactivity and bed diffusivity. Loo and Wong (2005) showed that diffusivity of the bed could be improved by choosing a suitable coke particle size range.
Figure 4a illustrates the effect of coke particle size on the temperature rise rate of the sintering bed. As shown, the temperature rise rate is significantly higher for the CPR3 specimen than for the other samples with different coke particle sizes. Therefore, the maximum rate of temperature rise is achieved by removing the particles smaller than 0.212 mm and larger than 3.350 mm.
Maximum temperature of the sintering bed
Due to its correlation with the coke reactivity, the particle size distribution of coke could have a significant effect on the amount of heat produced as well as the maximum temperature of the sintering charge. Figure 4b shows the maximum temperatures observed in the middle thermocouple during the sintering of different specimens. This figure illustrates that the CPR3 specimen provided the highest temperature (1290±5°C) among the samples studied. Enhancement of the coke reactivity and bed diffusivity might be considered as the main reason for obtaining this range of temperature in the CPS3 specimens. However, previous studies show that high flame front temperatures are not always beneficial to productivity. Raising the flame front temperature increases airflow resistance and leads to increased sintering time and reduced productivity (Umadevi et al., 2011). Therefore, for the CPR3 samples, which provide a high flame front temperature due to the increased coke reactivity, the percentage of coke could be decreased to maintain the airflow at the optimal level.
Flame front velocity
In order to quantify the flame front velocity (V), it was assumed that the maximum temperature is produced in the flame front layer. Thus, as shown in Figure 3, the flame front velocity could be calculated as dX/dt; where X is the distance between the first and third thermocouples, and t is the time for each thermocouple to reach its maximum temperature. The flame front velocities obtained for various specimens are presented in Figure 4c. It can be seen that by removing the large coke particles, which possess little reactivity, and the small coke particles, which burn too fast and also reduce the bed diffusivity, the flame front velocity could be increased.
Among the experimental specimens, the CPR3 sample provides the maximum flame front velocity; therefore, due to the negative correlation between the flame front velocity and the sintering time, the abovementioned result confirms again that the shortest sintering time is obtained in the CPR3 sample. Based on this fact, using the coke particle size between 0.212 mm and 3.350 mm could increase the productivity of the sintering process.
Tumbler index (TI)
The cold strength is another important characteristic of the sinters that has a significant relationship with the amount of molten phase produced in the sintering bed. On the other hand, the amount of molten phase formed depends directly on the maximum temperature of flame front and consequently, it is greatly dependent on the coke reactivity as well as the bed diffusivity. Figure 4d presents the obtained TI values of the specimens with different coke particle sizes. According to this figure, although the CPS3 specimen had the maximum cold strength, its TI value is not significantly different from those of the CPS4 and CPS5 specimens. This fact may result from the proximity of maximum temperatures obtained in these three specimens, which produce similar amounts of molten phases during the sintering process.
Based on the composition of the raw materials as well as the CaO-FeOx-SiO2-Al2O3 phase diagram, which is illustrated in Figure 5 (the influence of MgO and MnO on the slag composition was neglected due to their small amounts in the mixture), the minimum temperature needed for molten phase formation in the sintering bed is around 1300°C (in the FeOx-CaO-SiO2 and FeOx-CaO-Al2O3 ternary systems). Therefore, due to the proximity of this temperature to the maximum temperatures obtained in the CPS3, CPS4, and CPS5 specimens (1290±5°C, 1272±4°C, and 1268±5°C, respectively), a molten phase is likely to be produced in these specimens, resulting in an increased TI value.

It is important to note that the increase of the liquid fraction has a negative impact on the bed diffusivity and thus may increase the sintering time. However, in the CPR3 sample, the negative effect of liquid phase on diffusivity is probably compensated by better distribution of the molten phase in the sintering pot, which reduces the preferred routes for airflow through the bed. Figure 6 shows two typical microstructures observed in the CPS1 and CPS3 specimens. This figure shows a number of preferred routes for airflow in the microstructure of CPS1, whereas using a relatively more suitable coke particle size in the CPS3 specimens results in a more homogeneous structure with well-distributed microp-orosities.

Conclusion
In this research, the effect of coke particle size on thermal profile, cold strength, and productivity of the sintering process was investigated. Results showed that the coke particle size range of 0.212-3.350 mm is the optimal range, which increases the heat production and temperature rise rates, maximum temperature, flame front velocity, and cold strength of the sinter. In fact, elimination of the particles smaller than 0.212 mm and larger than 3.350 mm creates an optimal condition for bed diffusivity and coke reactivity. However, it is clear that these results are valid for the experimental conditions applied in this study (e.g. the chemical composition, particle size of other ingredients, ignition temperature, sintering bed geometry, etc.), and might vary slightly under different experimental conditions.
Acknowledgement
We would like to acknowledge the contribution of the Esfahan Steel Company (ESCO) for providing the materials and equipment needed for this research.
References
Bhagat, R., Chattoraj, U., Goswami, M., Singh, D., and Sil, S. 2007. Effect of size parameters of mix ingredients on the porosity and reduction characteristics of sinter. Steel Research International, vol. 78, no. 6. pp. 451-454. [ Links ]
Chaigneau, R. 1994. Complex calcium ferrites in the blast furnace process: fluxed sinter formation and SFCA reduction under simulated conditions. PhD thesis, Delft University, The Netherlands. [ Links ]
Hosotani, Y., Konno, N., Yamaguchi, K., Orimotot, T., and Inazumi, T. 1996. Reduction properties of sinter with fine dispersed pores at high temperatures of 1273K and above. ISIJ International, vol. 36, no. 12. pp. 1439-1447. [ Links ]
KASAI, E., RANKIN, W. J., and GANNON, J. F. 1989. The effect of raw mixture properties on bed permeability during sintering. ISIJ International, vol. 29, no. 1. pp. 33-42. [ Links ]
Loo, C.E. and Wong, D. 2005. Fundamental factors determining laboratory sintering results. ISIJ International, vol. 45, no. 4. pp. 449-458. [ Links ]
Loo, C.E. 2005. A perspective of goethitic ore sintering fundamentals. ISIJ International, vol. 45, no. 4. pp. 436-448. [ Links ]
Lwamba, E. and Garbers-Craig, A.M. 2008. Control of the grain size distribution of the raw material mixture in the production of iron sinter. Journal of the South African Institute of Mining and Metallurgy, vol. 108, no. 5. pp. 293-299. [ Links ]
Mohamed, F., El-Hussiny, N., and Shalabi, M. 2010. Granulation of coke breeze fine for using in the sintering process. Science of Sintering, vol. 42, no. 2. pp. 193-202. [ Links ]
Nyembwe, M. 2011. Study of sinter reactions when fine iron ore is replaced with coarse ore, using an infrared furnace and sinter pot tests. Masters Dissertation, University of Pretoria, South Africa. [ Links ]
Ramos, M. V., Kasai, E., Kano, J., and Nakamura, T. 2000. Numerical simulation model of the iron ore sintering process directly describing the agglomeration phenomenon of granules in the packed bed. ISIJ International, vol. 40, no. 5. pp. 448-454. [ Links ]
Raygan, S., Abdizadeh, H., Dabbagh, A., and Pourabdoli, M. 2009. Influence of talc additive on cold strength and reducibility of iron ore sinters compared to bentonite. Ironmaking and Steelmaking, vol. 36, no. 4. pp. 273-278. [ Links ]
Teo, C., Mikka, R., and Loo, C. 1992. Positioning coke particles in iron ore sintering. ISIJ International, vol. 32, no. 10. pp. 10471057. [ Links ]
Umadevi, T., Brahmacharyulu, A., Roy, A. K., Mahapatra, P.C., Prabhu, M., and Ranjan, M. 2011. Influence of iron ore fines feed size on microstructure, productivity and quality of iron ore sinter. ISIJ International, vol. 51, no. 6. pp. 922-929. [ Links ]
Yang, W., Ryu, C., Choi, S., Choi, E., Lee, D., and Huh, W. 2004. Modeling of combustion and heat transfer in an iron ore sintering bed with considerations of multiple solid phases. ISIJ International, vol. 44, no. 3. pp. 492-499. [ Links ]
Paper received Oct. 2012
Revised paper received Oct. 2013
© The Southern African Institute of Mining and Metallurgy, 2013. ISSN 2225-6253.














